��Ŀ����
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�

������ͼ�����������գ�
��1���ڵ������У����Դ���������ĵ缫����������Ӧ�����ӷ���ʽΪ
�������������������������������������������������������������������� ��
���Դ���������ĵ缫��������ҺpH�������� ��ѡ����䣬�����½���
��2����ҵʳ�κ�Ca2+��Mg2+�����ʡ����ƹ��̷�����Ӧ�����ӷ���ʽΪ
������������������������ ���������������������������������������� ��
��3�����������SO42�������ϸߣ��������ӱ��Լ���ȥSO42�����ñ��Լ�������
���������������� ��ѡ��a��b��c����
a��Ba(OH)2�������������������� b��Ba(NO3)2�������������������� c��BaCl2
��4��Ϊ��Ч��ȥCa2+��Mg2+��SO42���������Լ��ĺ���˳�������������������� ��ѡ��a��b��c����
a���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
b���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c���ȼӱ��Լ������NaOH���ټ�Na2CO3
��5�����ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ������ ����ȴ������
����д�������ƣ���ȥNaCl��
��6���ڸ�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����������NaClO��H2����Ӧ�Ļ�ѧ����ʽΪ������������������������������������������������ ��
������
��1��2Cl�D�D2e��Cl2�������� ��2��Ca2+��CO Mg2+��2OH����Mg(OH)2�� ��3��a c ��4��b c ��5������������ ��6��NaCl��H2O
|
��ʾ��
�������У����Դ�����������ǵ����е�����������������Ӧ���������ӱ����������������仯ѧ����ʽΪ2Cl�D�D2e��Cl2�������Դ���������ĵ缫Ϊ�����������ӷ�����ԭ��Ӧ��ˮ�ĵ���ƽ�������ƻ���ˮ��һ�����룬�ü�������Һ�е�����������Ũ�ȴ���������Ũ�ȣ���Һ��pH����7�� �����к��в��������ʣ�ͨ���ܽ⡢���ˣ���ȥ���������ʡ���Һ�л����ڸơ�þ����������������ӣ���Σ���Dz���������������·��������þ����ʴ�缫����������������Լ���ʹ��������ת��Ϊ�������˳�ȥ��һ��Ϊ�˳����������ӣ������Լ���Ҫ�Թ������������Լ����轫ǰ��������Լ���ȥ����˲������Լ�����˳�����⡣��ȥþ���ӻ���������ӣ�ѡ���Լ���ͬ����䲻Ӱ�죬���ǰ��˳����Եߵ��������Լ���˳��Ҳ��һ����Ψһ�ġ����ӱ��Լ���ȥ��������ӣ���Ӧ��������ֻ���ǵ���ԭ�ϺͲ�Ʒ�йص������ӻ����������ӣ����������Թ����ı��Լ�����Ȼ����Һ�������˱��������ʣ���������̼������ӳ�ȥ�����ı����ӣ���Һ���Թ�����̼������ӿ��������ȥ��b��c��Ϊ��ȷѡ� �����£�����ʳ��ˮ��Ũ��ԼΪ26.5%��������ҺΪ16%���Ȼ�����10%���������ƵĻ����Һ�����������Ʒ�������ƣ���ɸ���һ����Ҫ��Ϣ�������Һ�����Σ����50%������������Һ������ʳ�η��ص��Һ������⡣����ȡ100 g�����Һ������10 g�������ơ�16 g�Ȼ��ƺ�74 gˮ�����������Ƶ�Ũ�ȴ�50%ʱ��ʣ���������ʵ�������Ҳ����10 g�������Ծ����ȡ�������������60��˵�ˮ������ȴ�����ˣ������ܼ��ļ��٣��Ȼ��ƴ���������������ҺΪ50%����������Һ�����پ��ؽᾧ����ô������������ơ� ��ҵ��ⱥ��ʳ��ˮ���豸����ʽ��Ĥ���ۣ�ʯ����Ĥ�����������������ֿ�������������������������������������������������������������������ơ�������Ĥ��ȥ���������ɵ��������������Ʒ�Ӧ���ɴ������ƺ��Ȼ��ƣ�ԭ�У�����˲�ƷΪ�����ᣨ����������ˮ��Һ�׳�ƯҺ������������Ӧ����ʽΪ��NaCl��H2O
|

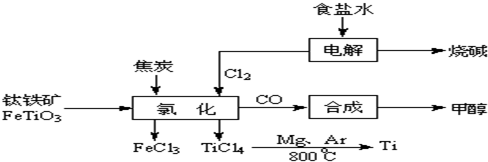
 2OH-+H2��+Cl2��
2OH-+H2��+Cl2�� 2MgCl2��s��+Ti����Ar�����н��е������ǣ�
2MgCl2��s��+Ti����Ar�����н��е������ǣ�





 ____________
____________

 ��Ar������������________
��Ar������������________