��Ŀ����
���бȽ��У���ȷ����
c��Na ��+c��H
��+c��H ��=c��S
��=c��S ��+c��HS
��+c��HS ��+c��OH
��+c��OH ��
��
| A��ͬ�¶�ͬ���ʵ���Ũ��ʱ��HF��HCN���룬��NaF��Һ��pH��NaCN��Һ�� |
B��0��2 mol ��L NH4Cl��0��1 mol��L NH4Cl��0��1 mol��L NaOH��Һ�������Ϻ� NaOH��Һ�������Ϻ�c��NH 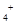 ��>c��Cl ��>c��Cl ��>c��Na ��>c��Na ��>c��OH ��>c��OH ��>c��H ��>c��H �� �� |
| C��ͬŨ�ȵ�������Һ�У���NH4Al��SO4��2����NH4Cl����CH3COONH4����NH3��H2O�� c��NH 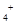 ���ɴ�С��˳����:��>��>��>�� ���ɴ�С��˳����:��>��>��>�� |
| D�����ʵ���Ũ����ȵ�H2S��HaHS�����Һ�У� |
 ��+c��H
��+c��H ��=c��S
��=c��S ��+c��HS
��+c��HS ��+c��OH
��+c��OH ��
��C
A����ͬ�¶�ͬ���ʵ���Ũ��ʱ��HF��HCN���룬������HF��HCN����NaF��Һ��pH��NaCN��ҺС��B������NH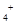 ����Ҫ����ˮ�⣬��Cl
����Ҫ����ˮ�⣬��Cl ���Ӳ�ˮ�⣬����c��NH
���Ӳ�ˮ�⣬����c��NH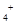 ����c��Cl
����c��Cl ����C��ȷ��D�����ɵ���غ�ɵ�c��Na
����C��ȷ��D�����ɵ���غ�ɵ�c��Na ��+c��H
��+c��H ��=2c��S
��=2c��S ��+c��HS
��+c��HS ��+c��OH
��+c��OH ��
��
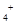 ����Ҫ����ˮ�⣬��Cl
����Ҫ����ˮ�⣬��Cl ���Ӳ�ˮ�⣬����c��NH
���Ӳ�ˮ�⣬����c��NH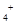 ����c��Cl
����c��Cl ����C��ȷ��D�����ɵ���غ�ɵ�c��Na
����C��ȷ��D�����ɵ���غ�ɵ�c��Na ��+c��H
��+c��H ��=2c��S
��=2c��S ��+c��HS
��+c��HS ��+c��OH
��+c��OH ��
��
��ϰ��ϵ�д�
�����Ŀ
 ������0.1mol��L-1AlCl3��Һ�У�Ag+�����ʵ���Ũ�����ɴﵽ
������0.1mol��L-1AlCl3��Һ�У�Ag+�����ʵ���Ũ�����ɴﵽ mol��L-1
mol��L-1
 H����CO3
H����CO3 2����H2O
2����H2O 