��Ŀ����
ij�о���ѧϰС�齫һ��Ũ��Na2CO3��Һ����CuSO4��Һ�еõ���ɫ��������ͬѧ��Ϊ���߷�Ӧֻ����CuCO3һ�ֳ�������ͬѧ��Ϊ��������ٽ�ˮ�ⷴӦ������Cu(OH)2һ�ֳ�������ͬѧ��Ϊ����CuCO3��Cu(OH)2���ֳ����� ����������֪��CuCO3��Cu(OH)2�������ᾧˮ��
��.������ͬѧ������Na2CO3��Һ��CuSO4��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ�� _______________________________�� ��̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ �ٹ��ˢ�ϴ�Ӣ۸��
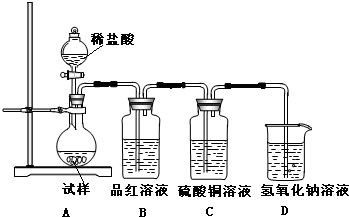
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
��.������ͬѧ������Na2CO3��Һ��CuSO4��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ�� _______________________________�� ��̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ �ٹ��ˢ�ϴ�Ӣ۸��
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�

��1����װ������˳��Ϊ __________________��
��2��װ��C��װ�е��Լ�������____________________��
��3����֤������������CuCO3��ʵ��������_________________��
����CuCO3��Cu(OH)2���߶��У���ͨ��������ʾװ�ý��ж����������ⶨ����ɡ�
��2��װ��C��װ�е��Լ�������____________________��
��3����֤������������CuCO3��ʵ��������_________________��
����CuCO3��Cu(OH)2���߶��У���ͨ��������ʾװ�ý��ж����������ⶨ����ɡ�

��1����װ������˳��Ϊ_____________��
��2��װ��C�м�ʯ�ҵ������� __________________��
��3����������Ʒ������Ϊm�ˣ�װ��B����������n�ˣ��������Cu(OH)2����������Ϊ
_______________��
��2��װ��C�м�ʯ�ҵ������� __________________��
��3����������Ʒ������Ϊm�ˣ�װ��B����������n�ˣ��������Cu(OH)2����������Ϊ
_______________��
�� Na2CO3 +CuSO4 +H2O=Cu(OH)2��+Na2SO4+CO2��
��1��A��C��B����2����ˮ����ͭ����3��װ��B�г���ʯ��ˮ�����
��1��CABDE��CABED����2�����տ����е�ˮ������CO2�� ��3��49n/9m
��1��A��C��B����2����ˮ����ͭ����3��װ��B�г���ʯ��ˮ�����
��1��CABDE��CABED����2�����տ����е�ˮ������CO2�� ��3��49n/9m

��ϰ��ϵ�д�
�����Ŀ


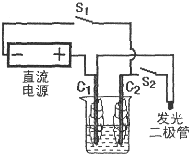 ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��
ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��