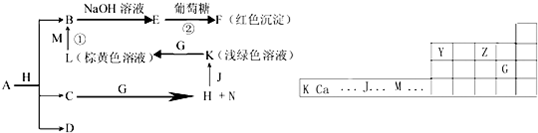
��Ŀ����
�����£�������������Һ��
| �� | �� | �� | �� |
| 0.1mol/L NaOH��Һ | pH=11 NaOH��Һ | 0.1mol/L CH3COOH��Һ | pH=3 CH3COOH��Һ |
����˵����ȷ����
- A.��ˮ�������c(H+)����>��
- B.��ϡ�͵�ԭ����100����pH�����ͬ
- C.����ۻ�ϣ�����ҺpH=7����V(NaOH)>V(CH3COOH)
- D.����ܻ�ϣ�����Һ�����ԣ���������Һ������Ũ�ȿ���Ϊ��c(CH3COO��)>c(H+)>c(Na+)>c(OH��)
AD
���������NaOH��ǿ�CH3COOH�����ᣬ���Ƕ���ʹˮ�ĵ���ƽ�⣨H2O H++OH�������ƣ���ǰ��ʹˮ�ĵ���ƽ�����Ƶij̶ȴ��ں��ߣ�����ˮ�������������Ũ�ȸ�С����A��ȷ������ϡ�Ͷ��ɣ�c1?V1=c2?V2�����ϡ�͵�ԭ����100�������Ũ�ȱ�Ϊ0.001mol/L�����Ǵ��������ᣬ��ʹϡ��100��Һ������ȫ���룬����Һ��������Ũ��С��0.001mol/L������pH=��lgc(H+)������ҺpH>3�������������ͬ����B����������۵������ϣ�����n=c?V��֪��NaOH��CH3COOHǡ����ȫ�кͣ��õ���CH3COONa��ǿ�������Σ���Ϻ���Һ�������ԣ�pH>7������Һ��pH=7�������һ�����������V(NaOH)<V(CH3COOH)����C��������Һ��������ƽ�⣺CH3COOH
H++OH�������ƣ���ǰ��ʹˮ�ĵ���ƽ�����Ƶij̶ȴ��ں��ߣ�����ˮ�������������Ũ�ȸ�С����A��ȷ������ϡ�Ͷ��ɣ�c1?V1=c2?V2�����ϡ�͵�ԭ����100�������Ũ�ȱ�Ϊ0.001mol/L�����Ǵ��������ᣬ��ʹϡ��100��Һ������ȫ���룬����Һ��������Ũ��С��0.001mol/L������pH=��lgc(H+)������ҺpH>3�������������ͬ����B����������۵������ϣ�����n=c?V��֪��NaOH��CH3COOHǡ����ȫ�кͣ��õ���CH3COONa��ǿ�������Σ���Ϻ���Һ�������ԣ�pH>7������Һ��pH=7�������һ�����������V(NaOH)<V(CH3COOH)����C��������Һ��������ƽ�⣺CH3COOH H++CH3COO��������������Һ�е�������NaOH��Һ�����������ӵ�������ƽ�����ƣ����������кͷ�Ӧʱ������ԶԶ������NaOH���ز��㣬��������Һ�п��ܳ��֣�c(CH3COO��)>c(H+)>c(Na+)>c(OH��)����D��ȷ��
H++CH3COO��������������Һ�е�������NaOH��Һ�����������ӵ�������ƽ�����ƣ����������кͷ�Ӧʱ������ԶԶ������NaOH���ز��㣬��������Һ�п��ܳ��֣�c(CH3COO��)>c(H+)>c(Na+)>c(OH��)����D��ȷ��
���㣺����ˮ��Һ�е�����ƽ�⣬�漰ǿ������ʡ�ˮ�ĵ���ƽ���ƶ���ϡ�Ͷ��ɵ�Ӧ�á�������ʵ���ƽ�⡢����ˮ�⡢��Һ���������pH�ȡ�
���������NaOH��ǿ�CH3COOH�����ᣬ���Ƕ���ʹˮ�ĵ���ƽ�⣨H2O
 H++OH�������ƣ���ǰ��ʹˮ�ĵ���ƽ�����Ƶij̶ȴ��ں��ߣ�����ˮ�������������Ũ�ȸ�С����A��ȷ������ϡ�Ͷ��ɣ�c1?V1=c2?V2�����ϡ�͵�ԭ����100�������Ũ�ȱ�Ϊ0.001mol/L�����Ǵ��������ᣬ��ʹϡ��100��Һ������ȫ���룬����Һ��������Ũ��С��0.001mol/L������pH=��lgc(H+)������ҺpH>3�������������ͬ����B����������۵������ϣ�����n=c?V��֪��NaOH��CH3COOHǡ����ȫ�кͣ��õ���CH3COONa��ǿ�������Σ���Ϻ���Һ�������ԣ�pH>7������Һ��pH=7�������һ�����������V(NaOH)<V(CH3COOH)����C��������Һ��������ƽ�⣺CH3COOH
H++OH�������ƣ���ǰ��ʹˮ�ĵ���ƽ�����Ƶij̶ȴ��ں��ߣ�����ˮ�������������Ũ�ȸ�С����A��ȷ������ϡ�Ͷ��ɣ�c1?V1=c2?V2�����ϡ�͵�ԭ����100�������Ũ�ȱ�Ϊ0.001mol/L�����Ǵ��������ᣬ��ʹϡ��100��Һ������ȫ���룬����Һ��������Ũ��С��0.001mol/L������pH=��lgc(H+)������ҺpH>3�������������ͬ����B����������۵������ϣ�����n=c?V��֪��NaOH��CH3COOHǡ����ȫ�кͣ��õ���CH3COONa��ǿ�������Σ���Ϻ���Һ�������ԣ�pH>7������Һ��pH=7�������һ�����������V(NaOH)<V(CH3COOH)����C��������Һ��������ƽ�⣺CH3COOH H++CH3COO��������������Һ�е�������NaOH��Һ�����������ӵ�������ƽ�����ƣ����������кͷ�Ӧʱ������ԶԶ������NaOH���ز��㣬��������Һ�п��ܳ��֣�c(CH3COO��)>c(H+)>c(Na+)>c(OH��)����D��ȷ��
H++CH3COO��������������Һ�е�������NaOH��Һ�����������ӵ�������ƽ�����ƣ����������кͷ�Ӧʱ������ԶԶ������NaOH���ز��㣬��������Һ�п��ܳ��֣�c(CH3COO��)>c(H+)>c(Na+)>c(OH��)����D��ȷ�����㣺����ˮ��Һ�е�����ƽ�⣬�漰ǿ������ʡ�ˮ�ĵ���ƽ���ƶ���ϡ�Ͷ��ɵ�Ӧ�á�������ʵ���ƽ�⡢����ˮ�⡢��Һ���������pH�ȡ�

��ϰ��ϵ�д�
�����Ŀ



 +NaHCO3��
+NaHCO3�� +CO2��+H2O
+CO2��+H2O
 ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2��C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�