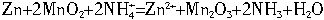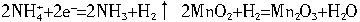��Ŀ����
п�̸ɵ�����ձ�ʹ�õĻ�ѧ��Դ�����к���MnO2��NH4Cl��ZnCl2�Ⱥ�״���пͲΪ�������ϣ�ʯīΪ�������ϡ�һ�ڸɵ�ص綯�ƺ��ڵ���ֱ�Ϊg=1.5V��r=0.25�����������ʱЧ�� =75%���ɵ�صĹ���ԭ���ǣ� Zn+2MnO2+2NH4+=Zn2++Mn2O3+2NH3��+ H2O
(1)��д���ɵ�طŵ�ʱ������������Ӧʽ������_____________������_____________��
(2)������Ӧ�У�ǰ����������������Ӧ��2NH4++2e-=2NH3��+H2�� 2MnO2+H2=Mn2O2+H2O ���������Ӧû��MnO2�IJ��룬�ɵ�ؽ����Գ����ȶ���������˵�����ɣ�____________________________________��
(3)��ͨ��10min�ڲμӷ�Ӧ��MnO2����ԼΪ���٣���������ĵĵ����Ƕ��٣�
______________________________
(4)������ҶԷϾɵ�ؽ��л��գ��ӱ��������ͽ�Լ��Դ�ĽǶȽ���ΪʲôҪ���շϾɵ�أ�__________________________
(1)��д���ɵ�طŵ�ʱ������������Ӧʽ������_____________������_____________��
(2)������Ӧ�У�ǰ����������������Ӧ��2NH4++2e-=2NH3��+H2�� 2MnO2+H2=Mn2O2+H2O ���������Ӧû��MnO2�IJ��룬�ɵ�ؽ����Գ����ȶ���������˵�����ɣ�____________________________________��
(3)��ͨ��10min�ڲμӷ�Ӧ��MnO2����ԼΪ���٣���������ĵĵ����Ƕ��٣�
______________________________
(4)������ҶԷϾɵ�ؽ��л��գ��ӱ��������ͽ�Լ��Դ�ĽǶȽ���ΪʲôҪ���շϾɵ�أ�__________________________
(1)2MnO2+2NH4++2e-=Mn2O3+2NH3��+ H2O��Zn-2e-=Zn2+
(2)������Ӧ�в�����H2������ʯī�ϣ����ӵ������
(3)0.813g��1.01��103J
(4)���Ի��յ������Ľ������Ϻͻ���ԭ�ϡ��Ͼɵ���ж���̬�����к�����������Ȼ�����¼��ѱ����⣻�Ͼ�п�̸ɵ�غ��и�Ũ�ȵ��Ȼ����Һ�����������л�ʹ�����ữ��������ˮ������
(2)������Ӧ�в�����H2������ʯī�ϣ����ӵ������
(3)0.813g��1.01��103J
(4)���Ի��յ������Ľ������Ϻͻ���ԭ�ϡ��Ͼɵ���ж���̬�����к�����������Ȼ�����¼��ѱ����⣻�Ͼ�п�̸ɵ�غ��и�Ũ�ȵ��Ȼ����Һ�����������л�ʹ�����ữ��������ˮ������

��ϰ��ϵ�д�
�����Ŀ
 �������ʱ��Ч��Ϊ?��=75%���ɵ�صĹ���ԭ���ǣ�
�������ʱ��Ч��Ϊ?��=75%���ɵ�صĹ���ԭ���ǣ�