��Ŀ����
1Lij�����Һ�����ܺ��е����������
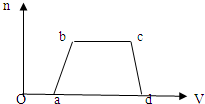
��1��������Һ����μ���NaOH��Һ���������������ʵ�����n�������NaOH��Һ�������V���Ĺ�ϵ��ͼ��ʾ�������Һ��
ȷ�����е�������______��һ�������е���������______��һ�������ڵ���������______��b��c�����ӷ���ʽΪ______��
��2������⣬����Һ�л����д�����Cl-��Br-��I-������1L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ�����ʾ��������ش��������⣺
�ٵ�ͨ��Cl2�����Ϊ2.8Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ______��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______��
| ���ܴ������е������� | H+��K+��Mg2+��Al3+��NH4+��Fe2+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��AlO2- |
ȷ�����е�������______��һ�������е���������______��һ�������ڵ���������______��b��c�����ӷ���ʽΪ______��

��2������⣬����Һ�л����д�����Cl-��Br-��I-������1L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ�����ʾ��������ش��������⣺
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n��Cl-�� | 1.25mol | 1.5mol | 2mol |
| n��Br-�� | 1.5mol | 1.4mol | 0.9mol |
| n��I-�� | amol | 0 | 0 |
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______��
��1������ͼ��ʼ����NaOHû�г����������������һ����H+�������г��������������ʧ����һ��û��Mg2+��Fe2+��Fe3+����Al3+��b��c�������������䣬ӦΪNH4++OH-=NH3?H2O�ķ�Ӧ������NH4+��������֪������K+����Ϊ��H+������CO32-��AlO2-��
�ʴ�Ϊ��H+��Al3+��NH4+��Mg2+��Fe2+��Fe3+��CO32-��AlO2-��NH4++OH-=NH3?H2O��
��2�����ڻ�ԭ�ԣ�I-��Br-����������2.8Lʱ��Һ��I-amol��
��˵��ͨ��2.8LCl2ֻ������Cl2+2I-=I2+2Cl-��
��ʱn��Cl2��=
=0.125mol������n��I-��=2��0.125mol=0.25mol������n��Cl-��=2��0.125mol=0.25mol��
��ʱn��Br-��=1.5mol����˵��ԭ��Һ��n��Br-��=1.5mol��
���ݱ����ݣ�2.8L���5.6L������2.8LCl2�����ʵ���Ϊ
=0.125mol��
Cl2 +2Br-=Br2+2Cl-
0.05mol 0.1mol
Cl2+2I-=I2+2Cl-
0.075mol0.15mol
��a=0.15mol������ԭ��Һ�У�
n��I-��=0.25mol+0.15mol=0.4mol��
n��Cl-��=1.25mol-0.25mol=1mol��
n��Cl-����n��Br-����n��I-��=1��1.5��0.4=10��15��4
�ʴ�Ϊ����Cl2+2I-=2Cl-+I2����10��15��4��
�ʴ�Ϊ��H+��Al3+��NH4+��Mg2+��Fe2+��Fe3+��CO32-��AlO2-��NH4++OH-=NH3?H2O��
��2�����ڻ�ԭ�ԣ�I-��Br-����������2.8Lʱ��Һ��I-amol��
��˵��ͨ��2.8LCl2ֻ������Cl2+2I-=I2+2Cl-��
��ʱn��Cl2��=
| 2.8L |
| 22.4L/mol |
��ʱn��Br-��=1.5mol����˵��ԭ��Һ��n��Br-��=1.5mol��
���ݱ����ݣ�2.8L���5.6L������2.8LCl2�����ʵ���Ϊ
| 2.8L |
| 22.4L/mol |
Cl2 +2Br-=Br2+2Cl-
0.05mol 0.1mol
Cl2+2I-=I2+2Cl-
0.075mol0.15mol
��a=0.15mol������ԭ��Һ�У�
n��I-��=0.25mol+0.15mol=0.4mol��
n��Cl-��=1.25mol-0.25mol=1mol��
n��Cl-����n��Br-����n��I-��=1��1.5��0.4=10��15��4
�ʴ�Ϊ����Cl2+2I-=2Cl-+I2����10��15��4��

��ϰ��ϵ�д�
 һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
�����Ŀ
