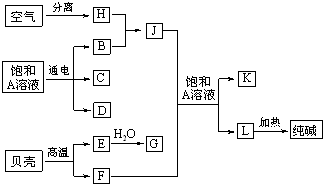
��Ŀ����
 �Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮
�Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮
��1��C��D��C��G��Ӧ��������ȡ��������C��D��Ӧ��ȡ������������Ч�ɷֵĻ�ѧʽΪ______��
��2�����A��Һ��Ӧ�����ӷ���ʽΪ______��
��3����F��Jͨ��A�ı�����Һ�У���Ӧ�Ļ�ѧ����ʽΪ��______��
��4��J�Ŀռ乹��Ϊ______�Σ�
��5�����������H��ʣ�����������Ҫ�ɷ���______���п��Խ�����B��D���ȼ�ϵ�أ���õ�صĸ�����Ӧʽ��______��������Ӧʽ��______��
�⣺���ǵ���Ҫ�ɷ�ΪCaCO3�����ȿ�����CaO��CO2���ɴ����֪LΪNaHCO3����F��J�ķ�ӦӦΪ�����Ƽ�ķ�Ӧ���ɴ˿�֪EΪCaO��FΪCO2��GΪCa��OH��2��JΪNH3��AΪNaCl��KΪNH4Cl��HΪN2��BΪH2��C��D��C��G��Ӧ��������ȡ����������CΪCl2��DΪNaOH��
��1�������Ϸ�����֪CΪCl2��DΪNaOH�����߷�Ӧ����NaCl��NaClO������NaClOΪ����������Ч�ɷ֣��ʴ�Ϊ��NaClO��
��2��AΪNaCl����ҵ�������ӷ���ʽΪ2H2O+2Cl- Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-��
�ʴ�Ϊ��2H2O+2Cl- Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-��
��3����F��Jͨ��A�ı�����Һ�У�Ϊ�����Ƽ�ķ�Ӧ����Ӧ�ķ���ʽΪNaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl��
�ʴ�Ϊ��NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl��
��4��JΪNH3��Ϊ�����νṹ���ʴ�Ϊ��������
��5��HΪN2�����������H��ʣ�����������Ҫ�ɷ���O2��BΪH2��DΪNaOH����ɼ���ȼ�ϵ�أ���������������Ӧ���缫��ӦʽΪ2H2+4OH--4e-�T4H2O������������ԭ��Ӧ���缫��ӦʽΪO2+2H2O+4e-�T4OH-��
�ʴ�Ϊ��O2��2H2+4OH--4e-�T4H2O��O2+2H2O+4e-�T4OH-��
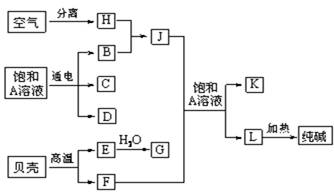
���������ǵ���Ҫ�ɷ�ΪCaCO3�����ȿ�����CaO��CO2���ɴ����֪LΪNaHCO3����F��J�ķ�ӦӦΪ�����Ƽ�ķ�Ӧ���ɴ˿�֪EΪCaO��FΪCO2��GΪCa��OH��2��JΪNH3��AΪNaCl��KΪNH4Cl��HΪN2��BΪH2��C��D��C��G��Ӧ��������ȡ����������CΪCl2��DΪNaOH��������ʵ����ʺ���Ŀ��Ҫ������⣮
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע����ݱ��Ǻͺ����ƼΪ�����ͻ�ƿڽ����ƶϣ�ѧϰ��ע�����ԭ��صĹ���ԭ���͵缫��Ӧʽ����д��
��1�������Ϸ�����֪CΪCl2��DΪNaOH�����߷�Ӧ����NaCl��NaClO������NaClOΪ����������Ч�ɷ֣��ʴ�Ϊ��NaClO��
��2��AΪNaCl����ҵ�������ӷ���ʽΪ2H2O+2Cl-
 Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-���ʴ�Ϊ��2H2O+2Cl-
 Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-����3����F��Jͨ��A�ı�����Һ�У�Ϊ�����Ƽ�ķ�Ӧ����Ӧ�ķ���ʽΪNaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl��
�ʴ�Ϊ��NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl��
��4��JΪNH3��Ϊ�����νṹ���ʴ�Ϊ��������
��5��HΪN2�����������H��ʣ�����������Ҫ�ɷ���O2��BΪH2��DΪNaOH����ɼ���ȼ�ϵ�أ���������������Ӧ���缫��ӦʽΪ2H2+4OH--4e-�T4H2O������������ԭ��Ӧ���缫��ӦʽΪO2+2H2O+4e-�T4OH-��
�ʴ�Ϊ��O2��2H2+4OH--4e-�T4H2O��O2+2H2O+4e-�T4OH-��
���������ǵ���Ҫ�ɷ�ΪCaCO3�����ȿ�����CaO��CO2���ɴ����֪LΪNaHCO3����F��J�ķ�ӦӦΪ�����Ƽ�ķ�Ӧ���ɴ˿�֪EΪCaO��FΪCO2��GΪCa��OH��2��JΪNH3��AΪNaCl��KΪNH4Cl��HΪN2��BΪH2��C��D��C��G��Ӧ��������ȡ����������CΪCl2��DΪNaOH��������ʵ����ʺ���Ŀ��Ҫ������⣮
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע����ݱ��Ǻͺ����ƼΪ�����ͻ�ƿڽ����ƶϣ�ѧϰ��ע�����ԭ��صĹ���ԭ���͵缫��Ӧʽ����д��

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ
 �Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮
�Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮