��Ŀ����
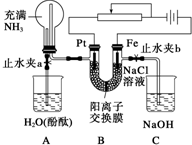
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
��������Ƿ������ش��������⣺
��1��д��Bװ���еĵ缫��Ӧ��
������______��
������______��
��2���۲쵽Aװ���е������ǣ�
��______��
��______��
��______��
��3�����۲쵽Aװ���е���������ǹر�ֹˮ��a����ֹˮ��b���ٹ۲�Cװ�ã�����������˵�����ɣ�����������д���йط�Ӧ�Ļ�ѧ����ʽ�������ӷ�Ӧ��д���ӷ���ʽ����______��
��4������ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��θĽ�װ�ã��������������______��
��������Ƿ������ش��������⣺
��1��д��Bװ���еĵ缫��Ӧ��
������______��
������______��
��2���۲쵽Aװ���е������ǣ�
��______��
��______��
��______��
��3�����۲쵽Aװ���е���������ǹر�ֹˮ��a����ֹˮ��b���ٹ۲�Cװ�ã�����������˵�����ɣ�����������д���йط�Ӧ�Ļ�ѧ����ʽ�������ӷ�Ӧ��д���ӷ���ʽ����______��
��4������ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��θĽ�װ�ã��������������______��

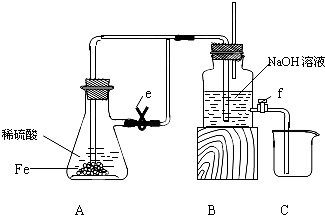
��1���ڵ��ص������ǻ��õ缫����ʧȥ���ӷ���������Ӧ��Fe-2e-�TFe2+���������ǵ�����е������������ӷ����õ��ӵĻ�ԭ��Ӧ����2H2O+2e-�TH2��+2OH-����2H++2e-�TH2�������ʴ�Ϊ��2H2O+2e-�TH2��+2OH-����2H++2e-�TH2������Fe-2e-�TFe2+��
��2���ڵ����У���Ϊ���������缫�ϲ��������������Ե���A�ձ�����ѹ��������ˮ�Ӵ���������������ˮ����ˮ������������ɫ��Ȫ������ƿ��Һ��������������ർ��һ���̶Ⱥ������������뵼����ƽʱ����ƿ����ѹǿ��ȣ���Ȫ������������ڰ�ˮ�Լ��ԣ��������A�ձ���Һ�ʺ�ɫ�������ڵ�������������������ʴ�Ϊ��A�ձ��е�ˮ������������ɫ��Ȫ����ƿ��Һ��������������ർ��һ���̶Ⱥ������������뵼����ƽ�����A�ձ���Һ�ʺ�ɫ�����������������
��3����Aװ���е���Ȫʵ��ر�ֹˮ��a����ֹˮ��b��Cװ���У������������ӵ��κ����е��������Ʒ�����Ӧ���������������������������ױ�����Ϊ�����������ɫ��������Fe2++2OH-�TFe��OH��2����4Fe��OH��2+2H2O+O2�T4Fe��OH��3��д���ܷ�Ӧʽ��4Fe2++8OH-+2H2O+O2�T4Fe��OH��3����
�ʴ�Ϊ��Fe2++2OH-�TFe��OH��2����4Fe��OH��2+2H2O+O2�T4Fe��OH��3��д���ܷ�Ӧʽ��4Fe2++8OH-+2H2O+O2�T4Fe��OH��3����
��4���ڵ�ⱥ��ʳ��ˮʱ��Ҫʵ�ֵ���Ȼ�����Һ��Ŀ�ģ�Ӧ��ѡ����Ե缫���е�⣬����Fe�缫����C��Pt�ȶ��Ե缫��װ�������缫��λ�õȣ�
�ʴ�Ϊ����Fe�缫����C��Pt�ȶ��Ե缫��װ�������缫��λ�õȣ�
��2���ڵ����У���Ϊ���������缫�ϲ��������������Ե���A�ձ�����ѹ��������ˮ�Ӵ���������������ˮ����ˮ������������ɫ��Ȫ������ƿ��Һ��������������ർ��һ���̶Ⱥ������������뵼����ƽʱ����ƿ����ѹǿ��ȣ���Ȫ������������ڰ�ˮ�Լ��ԣ��������A�ձ���Һ�ʺ�ɫ�������ڵ�������������������ʴ�Ϊ��A�ձ��е�ˮ������������ɫ��Ȫ����ƿ��Һ��������������ർ��һ���̶Ⱥ������������뵼����ƽ�����A�ձ���Һ�ʺ�ɫ�����������������
��3����Aװ���е���Ȫʵ��ر�ֹˮ��a����ֹˮ��b��Cװ���У������������ӵ��κ����е��������Ʒ�����Ӧ���������������������������ױ�����Ϊ�����������ɫ��������Fe2++2OH-�TFe��OH��2����4Fe��OH��2+2H2O+O2�T4Fe��OH��3��д���ܷ�Ӧʽ��4Fe2++8OH-+2H2O+O2�T4Fe��OH��3����
�ʴ�Ϊ��Fe2++2OH-�TFe��OH��2����4Fe��OH��2+2H2O+O2�T4Fe��OH��3��д���ܷ�Ӧʽ��4Fe2++8OH-+2H2O+O2�T4Fe��OH��3����
��4���ڵ�ⱥ��ʳ��ˮʱ��Ҫʵ�ֵ���Ȼ�����Һ��Ŀ�ģ�Ӧ��ѡ����Ե缫���е�⣬����Fe�缫����C��Pt�ȶ��Ե缫��װ�������缫��λ�õȣ�
�ʴ�Ϊ����Fe�缫����C��Pt�ȶ��Ե缫��װ�������缫��λ�õȣ�

��ϰ��ϵ�д�
�����Ŀ


 ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������