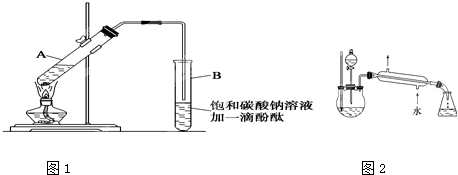
题目内容
已知下列数据

则下列热化学方程式不正确的是
[ ]
A、1/2H2(g)+1/2Cl2(g)==HCl(g) ;△H=-91.5kJ/mol
B、H2(g)+ Cl2(g)==2HCl(g) ;△H=-183kJ/mol
C、1/2H2(g)+1/2Cl2(g)==HCl(g) ;△H=+91.5kJ/mol
D、2HCl(g) ==H2(g)+ Cl2(g) ;△H=+183kJ/mol
B、H2(g)+ Cl2(g)==2HCl(g) ;△H=-183kJ/mol
C、1/2H2(g)+1/2Cl2(g)==HCl(g) ;△H=+91.5kJ/mol
D、2HCl(g) ==H2(g)+ Cl2(g) ;△H=+183kJ/mol
C

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
已知下列数据:
2Fe(s)+O2(g)=2FeO(s)△H=-544kJ?mol-1
4Al(s)+3O2(g)=2Al2O3(s)△H=-3350kJ?mol-1
则2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s)的△H是( )
2Fe(s)+O2(g)=2FeO(s)△H=-544kJ?mol-1
4Al(s)+3O2(g)=2Al2O3(s)△H=-3350kJ?mol-1
则2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s)的△H是( )
| A、-859 kJ?mol-1 | B、+859 kJ?mol-1 | C、-1403 kJ?mol-1 | D、-2491 kJ?mol-1 |
 某化学反应中,设反应物的总能量为E1,生成物的总能量为E2.
某化学反应中,设反应物的总能量为E1,生成物的总能量为E2.