��Ŀ����
�����仯������������������Ź㷺��Ӧ�á�
������ý(��2�����ͣ�3������ɵ�������)�ǹ�ҵ�ϳɰ��Ĵ�����ijѧϰС�����������̲ⶨ����ý�ĺ���������������ش�������⣺

��1�������������1.50 mol��L��1������100mL������18.4mol��L��1��Ũ���������ƣ�����Ҫ����Ͳ��ȡŨ���� mL
��2�������ͨ��Cl2��Ŀ���� �������ӷ���ʽ��ʾ��
��3������ܺ����� (����������)ȡ25.00mLϡ�ͺ���Һ��
��4�����������0.10mol��L��1��Na2SO3��Һ25.00mL��������ý��������������Ϊ ��
��Ŀǰ���о�����������ijЩ�������ο����ڹ�ҵ��ˮ�������Ĵ�����
��1����K2FeO4�������Է�ˮʱ��ˮ��Ӧ���������������壬���ų���ɫ��ζ��������������ʣ�д����Ӧ�����ӷ�Ӧ����ʽ ��������ˮʱ������K2FeO4ǿ�����ԣ������������������������ ���á�
��2��MFe2O4������������Ӧ�Ʊ��������ײ�����ȱλ������MFe2Ox (3<x<4)������M��ʾ+2�۵Ľ���Ԫ�أ������£�MFe2Ox��ʹ��ҵ�����е�SO2ת��ΪS���ﵽ������������Ŀ�ģ�ת�����̱�ʾ���£�

������ж�x y�������ԣ�MFe2Oy SO2�����������������=����
��1��8.2��2��2Fe2����Cl2��2Fe3����2Cl����3����ʽ�ζ���(����Һ��) ��4��35.7%
��1��4FeO42-+ 10H2O �� 4Fe(OH)3(����) +8OH�� + 3O2�����۳�������������2��������.
��������
���������������Һϡ��ǰ�����ʵ����ʵ������䣬����ϡ��ʽC1V1=C1V2�ɵ�1.50 mol��L��1��100m=18.4mol��L��1��Vml,���V=8.2ml. ��2�������ͨ��Cl2��Ŀ���ǰ�������������Ϊ�����ӣ����ӷ���ʽ��2Fe2����Cl2��2Fe3����2Cl����3���Ȼ�����Һ������Ҫ����Һ�ܻ���ʽ�ζ�������ȡ����ֹ��ʴ��ʽ�ζ��ܵ��ܡ���4��2Fe3++SO32-+H2O=2Fe2+SO42-+2H+������������������������ӵĹ�ϵ���õ�ԭ���Ĺ����к������������ʵ���Ϊ0.05mol������Ϊ0.05mol��6g/mol=2.8g.����������ý��������������Ϊ2.8g/7.84g��100�G=35.7%.��K2FeO4��ǿ�������ԣ���ɱ��������������������ԭΪ�����ӣ�����������Һ��ˮ����������������壬����������������ˮ�е�������ʹ֮��Ϊ�������Ӷ��ﵽ����ˮ��Ŀ�ġ�K2FeO4�������Է�ˮʱ��ˮ��Ӧ�����ӷ���ʽΪ��4FeO42-+ 10H2O �� 4Fe(OH)3(����) +8OH�� + 3O2��
���㣺���������仯��������������е�Ӧ�ü���Һϡ������ȵ�֪ʶ��

 һ����������ϵ�д�
һ����������ϵ�д� �����仯����������������й㷺Ӧ�ã���ش��������⣺
�����仯����������������й㷺Ӧ�ã���ش��������⣺
 ��2010?����һģ��FeԪ���ǵؿ�����ḻ��Ԫ��֮һ���ڽ����н��������������仯����������������й㷺Ӧ�ã���ش��������⣺
��2010?����һģ��FeԪ���ǵؿ�����ḻ��Ԫ��֮һ���ڽ����н��������������仯����������������й㷺Ӧ�ã���ش��������⣺

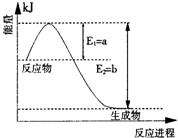
 6SO2��Fe3O4����3 molFeS2�μӷ�Ӧ��ת����������������mol���ӡ�
6SO2��Fe3O4����3 molFeS2�μӷ�Ӧ��ת����������������mol���ӡ�
 6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת��
mol���ӡ�
6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת��
mol���ӡ�