��Ŀ����
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ�����У�T��������������������������ȡ���ش���������

��1��T��ԭ�ӽṹʾ��ͼΪ____________________
��2��Ԫ�صķǽ����ԣ�ԭ�ӵĵõ�����������Q__________W���ǿ�ڡ������ڡ�����
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��
______________________��
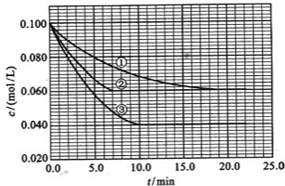
��5��R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û�����������������R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ��____________��
��6����298K�£�Q��T�ĵ��ʸ�1 mol��ȫȼ�գ��ֱ�ų�����a kJ��b kJ����֪һ�������£�T�ĵ����ܽ�Q������������������û������������û���Ӧ����3mol Q�ĵ��ʣ���÷�Ӧ��298K�µġ�H=
___________ ��ע���������浥�ʾ�Ϊ���ȶ����ʣ���
��2��Ԫ�صķǽ����ԣ�ԭ�ӵĵõ�����������Q__________W���ǿ�ڡ������ڡ�����
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��
______________________��
��5��R�ж�����������м���Է���������С����һ�������£�2L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û�����������������R�ĺ�������ֻ��һ�֣���ú������εĻ�ѧʽ��____________��
��6����298K�£�Q��T�ĵ��ʸ�1 mol��ȫȼ�գ��ֱ�ų�����a kJ��b kJ����֪һ�������£�T�ĵ����ܽ�Q������������������û������������û���Ӧ����3mol Q�ĵ��ʣ���÷�Ӧ��298K�µġ�H=
___________ ��ע���������浥�ʾ�Ϊ���ȶ����ʣ���
��1��
��2������
��3��S+2H2SO4��Ũ�� 3SO2��+2H2O
3SO2��+2H2O
��4��2H2O2 2H2O+O2��
2H2O+O2��
��5��NaNO2
��6��(3a-4b)kJ/moL

��2������
��3��S+2H2SO4��Ũ��
 3SO2��+2H2O
3SO2��+2H2O��4��2H2O2
 2H2O+O2��
2H2O+O2�� ��5��NaNO2
��6��(3a-4b)kJ/moL

��ϰ��ϵ�д�
�����Ŀ
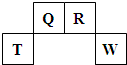 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
 ��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������
��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������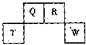 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺