��Ŀ����
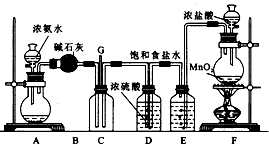
ijѧ����������װ��̽�������백��֮��ķ�Ӧ��ͼ��A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백����Ӧ��װ�á�
��ش��������⣺
(1)װ��A�е���ƿ�ڹ��岻����ѡ��__________��ѡ����ţ���
A.��ʯ��
B.��ʯ��
C.����������
D.�ռ�
(2)װ��F�з�����Ӧ�����ӷ���ʽΪ___________________________________��
(3)Bװ�õ�����___________��Eװ�õ�����___________��
(4)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ��____________________________________��
(5)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ�����___________________________________��
(1)װ��A�е���ƿ�ڹ��岻����ѡ��__________��ѡ����ţ���
A.��ʯ��
B.��ʯ��
C.����������
D.�ռ�
(2)װ��F�з�����Ӧ�����ӷ���ʽΪ___________________________________��
(3)Bװ�õ�����___________��Eװ�õ�����___________��
(4)װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ����д����Ӧ�Ļ�ѧ����ʽ��____________________________________��
(5)��װ��C��G���ݳ���β���п��ܺ��л���ɫ���ж����壬��δ�����___________________________________��
(1)C
(2)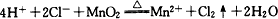
(3)���ﰱ������ȥ�����е��Ȼ���
(4)3Cl2+8NH3 = N2+6NH4Cl��3Cl2+2NH3 = N2+6HCl��NH3+HCl = NH4Cl
(5)��G���ӵ��ܣ�ֱ�Ӱ�β��ͨ��ʢ���ռ���Һ���ձ���
(2)
(3)���ﰱ������ȥ�����е��Ȼ���
(4)3Cl2+8NH3 = N2+6NH4Cl��3Cl2+2NH3 = N2+6HCl��NH3+HCl = NH4Cl
(5)��G���ӵ��ܣ�ֱ�Ӱ�β��ͨ��ʢ���ռ���Һ���ձ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ




