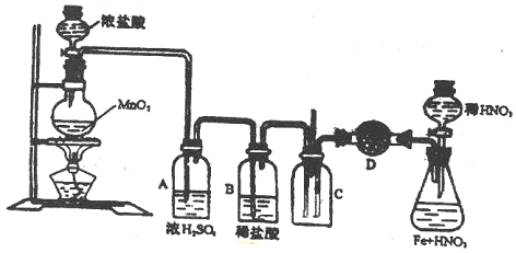
��Ŀ����
ʵ������������ͼ��װ�ú��Լ��ֱ���ȡCl2��H2S��ʹCl2���ᄏ�����H2S��Cװ���л�Ϸ�Ӧ��
(1)ָ��ͼ�������Լ�����װ����֮���������Ը�����_________________��
(2)A��Bƿ�е�Ũ�����ϡ�������ʲô���â�A��________ ��B��__________��
(3)�����D��ѡ��ʲôΪ�������
(4)C��������йط���ʽ��
(5)��C�����嵼�ܴ����������������� �����ݳ��������Ƿ���H2S��
(6)����A����D������H2S��������ĺ���������������� ���û�ѧ����ʽ��ʾ�������� ��
�𰸣�
������
��ʾ��
������
| (1)A��Bƿλ�õߵ���Ӧ�Ե���������Ӧ��ϡ���ᣬ��Ӧ��ϡ�����ϡ���ᡣ
(2)�����HCl���塣 (3)��ˮCaCl2���塣 (4)��������ɫ���壻H2S +Cl2== 2HCl+ S���� (5)ʪ��Ĵ���Ǧ��ֽ�� (6)H2S���ճ�ȥ�ﲻ����ʵ���Ŀ�ģ�H2S+H2SO4(Ũ)==S��+SO2��+2H2O��
|
��ʾ��
| ʵ������H2S���壬Ӧ����FeS��ϡ�����ϡ���ᷴӦ�����������ᣬ��Ϊ������ǿ�����ԣ��ὫH2S�������������Ƴ�H2S���塣ʵ���Ҹ���H2S����Ӧѡ�����Է������Ը��������ΪH2S�����Ժ�ǿ��ԭ�ԡ�����H2S���������ʪ��Ĵ���Ǧ��ֽ����ֽ���֤����H2S�� (CH3COO)2Pb+H2S==PbS��+2CH3COOH��������ͨ��CuSO4��Һ�У����ɺ�ɫ����˵����H2S���壨CuSO4+H2S==CuS��+H2SO4����
|

��ϰ��ϵ�д�
�����Ŀ