��Ŀ����
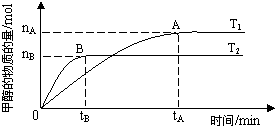
��ͼ��ʾ��4 mol��SO2��2 mol��O2�����������ɱ�ĵ�ѹ�����У���һ���¶��·������·�Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)����H��0���÷�Ӧ�ﵽƽ��״̬Aʱ��������������ʵ���Ϊ4.2 mol����SO2��O2��SO3����ʼ���ʵ����ֱ���a��b��c��ʾ���ش��������⣺
2SO3(g)����H��0���÷�Ӧ�ﵽƽ��״̬Aʱ��������������ʵ���Ϊ4.2 mol����SO2��O2��SO3����ʼ���ʵ����ֱ���a��b��c��ʾ���ش��������⣺

(1)�ڴﵽƽ��״̬����������ͨ������O2����ϵ��SO2���������________(�����С�����䡱)����ҪʹSO2����������ٱ䵽��ƽ��״̬A��ͬ���ɲ�ȡ�Ĵ�ʩ�У�________��________��
(2)����ʼʱa��1.2 mol��b��0.6 mol���Ҵﵽƽ������������������ƽ��״̬����ͬ������ʼʱc��ȡֵΪ________��
(3)��Ҫʹ��Ӧ��ʼʱ���淴Ӧ������У��Ҵﵽƽ������������ʵ�����ƽ��״̬A��ͬ������ʼʱc��ȡֵ��Χ��________��
������
����(1)��С�����¡�ͨ��������SO2
����(2)��0����ֵ
����(3)3.6��c��4

���»��籨����ȫ��ũ��Ӧ���ڡ���ɫ��̬��������ʣ���̼���ܣ�ѭ����չ�������������£�������õط�չ���й���ɫ��ׯ�������롰��̫���ʵ�̼ũׯ�����衣�ɼ�����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
��1��ú��������Һ���������ȼ�ϵ������ʡ�
��֪25�棬101kPaʱ��C(s) +1/2O2(g)=CO(g) ��H= -126.4 kJ��mol-1
2H2(g) +O2(g)=2H2O(l) ��H= -571.6 kJ��mol-1 H2O(g)= H2O(l) ��H= -44 kJ��mol-1
����25�棬101kPaʱ��C(s) + H2O(g)= CO(g) + H2(g) ��H=____________________��
��2����¯������CO�������Ҫ��;֮һ���������ӦΪ��
FeO(s)��CO(g) ![]() Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��Kֵ__________�����������С�����䡱����
��1100��ʱ��ø�¯�У�c(CO2)=0.025mol��L-1��c(CO)=0.1 mol��L-1��������������£���ƽ��_______�ƶ�������������ҡ����������ж�������
��3��Ŀǰ��ҵ�Ͽ���CO2������ȼ�ϼ״����йط�ӦΪ��
CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H��-49.0 kJ��mol-1���������Ϊ1 L���ܱ������У�����1mol CO2��3mol H2����Ӧ�����в��CO2��CH3OH(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H��-49.0 kJ��mol-1���������Ϊ1 L���ܱ������У�����1mol CO2��3mol H2����Ӧ�����в��CO2��CH3OH(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
|

 | ||||||||||||||
| ||||||||||||||
| ||||||||||||||
| ||||||||||||||
| ||||||||||||||
|
| |||||||||||||
| ||||||||||||||
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��_____________��
�����д�ʩ��ʹ![]() �������________������ţ���
�������________������ţ���
A�������¶� B���ٳ���H2 C���ٳ���CO2
D����H2O(g)����ϵ�з��� E������He(g)��ʹ��ϵѹǿ����
 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��

