��Ŀ����
����Ŀ���о��л���Ľṹ�Ǹ��õ�Ӧ���л����ǰ��
��1��![]() ��
��![]() �����л�����Ի��ܣ����й��������±���
�����л�����Ի��ܣ����й��������±���
����ܶ�/�� | �۵� | �е� | �ܽ��� | |
|
|
|
| ������ˮ |
|
|
|
| ��ˮ������Ȼ��� |
��Ҫ��ȥ![]() ��
��![]() �Ļ�����е�
�Ļ�����е�![]() ��������_________�������ɵõ��ϴ���
��������_________�������ɵõ��ϴ���![]() ��
��
A������ B���ؽᾧ C����ȡ D����ˮ�������Һ
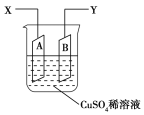
��Ϊ�ⶨ![]() �Ľṹ����������ʵ�飺��һ����
�Ľṹ����������ʵ�飺��һ����![]() �����������г��ȼ�գ�ʵ��������
�����������г��ȼ�գ�ʵ��������![]() ��
�� ![]() ����������6��72 L����״���£��������Dzⶨ���л�����Է�����Ϊ
����������6��72 L����״���£��������Dzⶨ���л�����Է�����Ϊ![]() ������л���ķ���ʽΪ__________��д��
������л���ķ���ʽΪ__________��д��![]() �����п��ܵĽṹ��ʽ____________________________________��
�����п��ܵĽṹ��ʽ____________________________________��
��������ͼ��ʾ![]() ����Է�������Ϊ
����Է�������Ϊ![]() �����������ʾ�жԳ�
�����������ʾ�жԳ�![]() ���Գ�
���Գ�![]() ����
����![]() ����
����![]() �Ľṹ��ʽΪ____________________________________��
�Ľṹ��ʽΪ____________________________________��
��2���ڳ����²ⶨ��Է�������Ϊ![]() ��ij���ĺ˴Ź������ף���Ϊ�������۲쵽�������͵�
��ij���ĺ˴Ź������ף���Ϊ�������۲쵽�������͵�![]() ԭ�Ӹ������źţ���ǿ��֮��Ϊ
ԭ�Ӹ������źţ���ǿ��֮��Ϊ![]() ����������Ľṹ��ʽΪ____________________________________����Ϊ���㣬��һ�ȴ�����
����������Ľṹ��ʽΪ____________________________________����Ϊ���㣬��һ�ȴ�����![]() �֣�������Ľṹ��ʽΪ______________________��
�֣�������Ľṹ��ʽΪ______________________��
��3��ijϩ��![]() ����Է�������Ϊ
����Է�������Ϊ![]() ��
��![]() ��
��![]() �����ӳɷ�Ӧ�õ�������ֻ�������ִ���˳���칹��ϩ��
�����ӳɷ�Ӧ�õ�������ֻ�������ִ���˳���칹��ϩ��![]() �������ӳɵõ������ϩ����˳ʽ�Ľṹ��ʽΪ________������Ϊ________��
�������ӳɵõ������ϩ����˳ʽ�Ľṹ��ʽΪ________������Ϊ________��
���𰸡� ![]()
![]()
![]() ��
��![]()
![]()
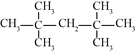
 ˳
˳![]() ��
��![]() ��
��![]() ��
��![]() �ļ�
�ļ�![]() ��ϩ
��ϩ
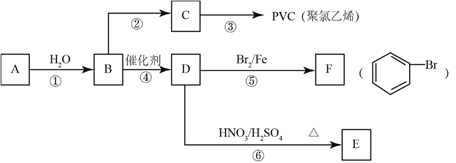
����������1�����ɱ������ݿ�֪��A������ˮ��B����ˮ����A��B���ܣ����߷е����ϴ�ȥA��B�Ļ�����е�����A�õ�B�ɲ��õķ���������ѡA����n��CO2��=![]() =0.2mol��n��H2O��=
=0.2mol��n��H2O��=![]() =0.3mol��n��O2��=
=0.3mol��n��O2��= ![]() =0.3mol�����л����к�n��O��=0.2mol��2+0.3mol-0.6mol=0.1mol�����л�����N��C����N��H����N��O��=0.2mol��0.6mol��0.1mol=2��6��1�����л���A��ʵ��ʽΪC2H6O������л���ķ���ʽΪ(C2H6O)n����Է�������Ϊ46������46n=46����õ�x=1������л���ķ���ʽΪC2H6O��B�����п��ܵĽṹ��ʽ��
=0.3mol�����л����к�n��O��=0.2mol��2+0.3mol-0.6mol=0.1mol�����л�����N��C����N��H����N��O��=0.2mol��0.6mol��0.1mol=2��6��1�����л���A��ʵ��ʽΪC2H6O������л���ķ���ʽΪ(C2H6O)n����Է�������Ϊ46������46n=46����õ�x=1������л���ķ���ʽΪC2H6O��B�����п��ܵĽṹ��ʽ��![]() ��
��![]() ���жԳ�
���жԳ�![]() �����жԳ�
�����жԳ�![]() ����
����![]() ��������Ϊ
��������Ϊ![]() ���պ���Է�����������74���������⣬��
���պ���Է�����������74���������⣬��![]() �Ľṹ��ʽΪ��
�Ľṹ��ʽΪ��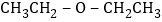 ����2��
����2��![]() =9
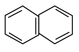
=9![]() 2���������Ӧ��ΪC9H20���˴Ź������ף��۲쵽�������͵�
2���������Ӧ��ΪC9H20���˴Ź������ף��۲쵽�������͵�![]() ԭ�Ӹ������źţ���ǿ��֮��Ϊ
ԭ�Ӹ������źţ���ǿ��֮��Ϊ![]() ������2��λ�õ�Hԭ�ӣ�����Ŀ�ֱ�Ϊ18��2�������Ľṹ��ʽΪ
������2��λ�õ�Hԭ�ӣ�����Ŀ�ֱ�Ϊ18��2�������Ľṹ��ʽΪ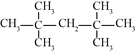 ����Ϊ���㣬
����Ϊ���㣬![]() =10
=10![]() 8������ʽΪC10H8�������Ľṹ��ʽΪ��
8������ʽΪC10H8�������Ľṹ��ʽΪ��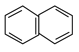 ����3��ijϩ��
����3��ijϩ��![]() ����Է�������Ϊ
����Է�������Ϊ![]() ��
��![]() =10�����ϩ��Ӧ��ΪC10H20��
=10�����ϩ��Ӧ��ΪC10H20��![]() ��
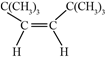
��![]() �����ӳɷ�Ӧ�õ�������ֻ�������ִ���˳���칹��ϩ��
�����ӳɷ�Ӧ�õ�������ֻ�������ִ���˳���칹��ϩ��![]() �������ӳɵõ������ϩ��˫��λ�����м��Ҹ߶ȶԳƣ��ṹ��ʽΪ��
�������ӳɵõ������ϩ��˫��λ�����м��Ҹ߶ȶԳƣ��ṹ��ʽΪ��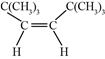 ������Ϊ˳
������Ϊ˳![]() ��
��![]() ��
��![]() ��
��![]() �ļ�
�ļ�![]() ��ϩ��
��ϩ��