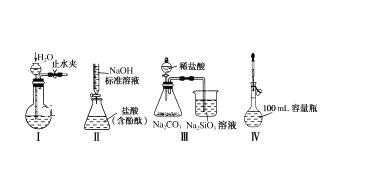
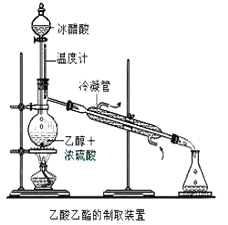
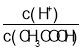��Ŀ����
����Ŀ��(1)��ɫֲ���ǿ�����Ȼ���������������ڼ����ѧѡ��һЩ��ɫֲ����Դﵽ���������������к����ʵ����á���ѧ�о����֣�1������ɼÿ�¿�������160 kg SO2����1������ɼÿ�����յ�SO2�����ʵ���Ϊ________��
(2)Ǧ��о����Ҫ�ɷ���ʯī���������Щ���ʰ��ղ�ͬ�ı������Ի�ϡ�ѹ�ƣ��Ϳ����Ƴɲ�ͬ�ͺŵ�Ǧ��о�����ijǦ��о������һ��ɷ���ʯī������Ǧ��дһ�������ĵ�����ԼΪ1 mg����ôһ��Ǧ���ֺ��е�̼ԭ����ԼΪ________��
(3)��ʳ��ҡͷ�����������ƻ��������������ҡͷ�����Ļ�ѧʽΪC9H13N������㣺
����ҡͷ������Ħ������Ϊ________��
��ij���ɱ�������270 g��ҡͷ�������ö�����������ҡͷ���������ʵ���Ϊ________��
(4)��14.3 g Na2CO3��10H2O��������ˮ���250 mL��Һ����������Һ���ʵ���Ũ����________��
���𰸡�2500 mol 2.5��1019 135g/mol 2 mol 0.2mol/L
��������
(1)����n=![]() ����SO2�����ʵ�����
����SO2�����ʵ�����
(2)����һ�������ĵ�����ԼΪ1 mg��Ǧ��о������һ��ɷ���ʯī������n=![]() ���������ʵ�����Ȼ������N=n��NA�������к��е�Cԭ����Ŀ��
���������ʵ�����Ȼ������N=n��NA�������к��е�Cԭ����Ŀ��
(3)�ٸ���Ħ����������Է���������ϵ���㣻
�ڸ���n=![]() ������ҡͷ���������ʵ�����
������ҡͷ���������ʵ�����
(4)����c=![]() ������Һ�����ʵ���Ũ�ȡ�
������Һ�����ʵ���Ũ�ȡ�
(1)160kgSO2�����ʵ���n=![]() =2500mol��
=2500mol��
(2)��һ������������ԼΪ1 mg��Ǧ��о������һ��ɷ���ʯī����һ�����к���Cԭ�ӵ����ʵ���Ϊn=![]() mol�������к��е�Cԭ����ĿΪN=nNA=
mol�������к��е�Cԭ����ĿΪN=nNA=![]() mol��6.02��1023/mol=2.5��1019��
mol��6.02��1023/mol=2.5��1019��
(3)��C9H13N����Է�������Ϊ��12��9+1��13+14=135����Ħ��������g/molΪ��λʱ����ֵ�ϵ�����Է��������������ҡͷ������Ħ������Ϊ135g/mol��
��270g��ҡͷ���������ʵ���n=![]() =2mol��
=2mol��
(4)14.3gNa2CO3��10H2O�����ʵ���n=![]() =0.05mol�����ø�̼���ƾ���������Һʱ������ΪNa2CO3�����ʵ����ʵ���n(Na2CO3)=0.05mol����������ʵ���Ũ�ȶ���ʽ��֪������Һ�����ʵ���Ũ��c=
=0.05mol�����ø�̼���ƾ���������Һʱ������ΪNa2CO3�����ʵ����ʵ���n(Na2CO3)=0.05mol����������ʵ���Ũ�ȶ���ʽ��֪������Һ�����ʵ���Ũ��c=![]() =0.2mol/L��
=0.2mol/L��