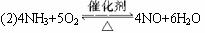��Ŀ����
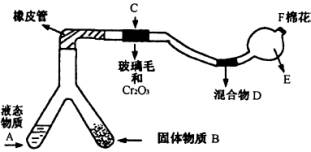
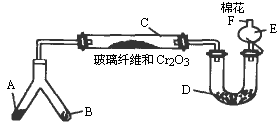
�ͻ�ѧʵ�����װ��С�ɣ���ԼҩƷ��������㣬�������ԣ���ȫ�ɿ���������Ⱦ���ص㣬��ͼ��ijͬѧ��Ƶ�NH3�Ĵ����������鷴Ӧ���ɵ��������ʵ���װ�ã�ͼ�б�Ҫ������̨�����С��;ƾ��ƵȾ���ȥ����
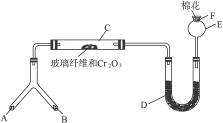
ʵ���������Լ����������з�Χ��
��NH4Cl��Ca(OH)2�Ļ���� ��4��1��ˮ ��NaOH���� ��KClO3��MnO2�Ļ���� ������ˮ��NH4HCO3���� ��6 mol��L-1 NaOH��Һ ��0.5 mol��L-1 NaOH��Һ ���̪��Һ ���ʯ�� 11ŨH2SO4 ?12CuO
������������⣺
��1���Ͳ��ι���Һ̬����A��__________����������B��__________��
��2��C��������Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��3�������D��Ϊ�˼����������ʵ����ɶ�����ģ��������__________��ʵ������е�������_______________________��
��4��E�����β����ܵ�������________________________________________��
��5��F������Ӧպȡ��������_________________________________���䷴Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��
��1��4��1�İ�ˮ����ڣ� KClO3��MnO2�Ļ�����ܣ�
(2)4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
(3)0.5 mol��L-1 NaOH��Һ�ͷ�̪��Һ�����͢ᣩ ��ɫ��ȥ
��4����ֹҺ̬����D���
��5��6 mol��L-1 NaOH��Һ����ߣ� NO2+NO+2NaOH====2NaNO2+H2O
����:
(1)��Ϊ��װ���ǰ��Ĵ����������鷴Ӧ���ɵ��������ʵ���װ�ã�����A��B��һ������O2�ģ�һ�����ư����ģ�������Ŀ��ʾBΪ��̬װ�ã������Լ���ֻ�Т�Ϊ��������ҩƷ��Ϊ��̬������B����KClO3��MnO2����AΪ�ư�����װ�á���ΪAΪҺ̬���ʣ�ֻ��ѡ�Լ��еĢڣ���4��1�İ�ˮ��ΪʹNH3�����ݳ���������NaOH���壩��
��2��������ά�����������NH3��O2�������ĽӴ������ʹ��Ӧ��ֵ����á�
��3��Ϊ�������ɵ��������壬����D�м����Һ�ͷ�̪��Һ��Ϊʹ��������ԣ�Ӧ����0.5 mol��L-1��NaOH��Һ������D�м���0.5 mol��L-1��NaOH��Һ�ͷ�̪��Һ�����Һ�ʺ�ɫ����

 ��У����ϵ�д�
��У����ϵ�д�