��Ŀ����
�廯��(CaBr2��2H2O)��һ�ְ�ɫ���壬������ˮ���к�ǿ����ʪ�ԣ��ǹ���ֽ��Ȫˮ����������Ҫ�ɷ֣���ҽҩ������������˥���ȵ�ҩ�Ҳ������ѧ�������ù�ҵ����ʯ����������Al3+��Fe3+�����ʣ��Ʊ��廯�Ƶ���Ҫ��������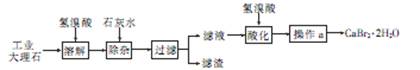
�ش���������
��1���ܽ�ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ
��2�����Ӳ��������Һ��pHԼΪ8��0��Ŀ���� ��
��3����Һ���������ữ��Ŀ���� ������a��Ҫ���� �� ����
��4���Ƶõ��廯�ƾ������ͨ�����²���ⶨ�䴿�ȣ�
�ٳ�ȡ5��00g�廯�ƾ�����Ʒ�����ܽ⣻�۵�������Naa2CO3��Һ����ַ�Ӧ����ˣ��ܺ�ɡ���ȴ���ݳ��������õ�2�� 00 g̼��ƣ�����Ʒ�Ĵ���Ϊ
��5���廯�ƾ����������Ӻ����ӵļ���
�ٽ������廯�ƾ�������ˮ�����������ữ��AgNO3��Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
�ڽ������廯�ƾ�������ˮ���μӲ�������Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
��1��CaCO3��2H��=Ca2����CO2����H2O��2�֣�
��2��ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣨��1�֣�
��3����ȥ������Ca��OH��2������Ũ������ȴ�ᾧ����1�֣�
��4��94��4%����0��944����2�֣�
��5���ٲ�������ɫ���ǣ�1�֣���Br����Ag��=AgBr����2�֣�
�ڲ�����ɫ������1�֣���Ca2++C2O42-=CaC2O4����2�֣�
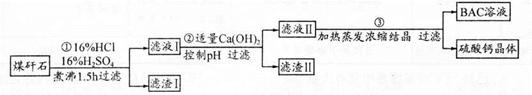
�������������������ѧ����������Ҫע�⣺������ͷ����ȷ����������Ŀ�Ģ������̽�������Ϣ��ȷÿһ����ԭ����Ŀ�ĺͲ����۴���Ҫע��淶��ע�⻯ѧ�������дҪ��ʵ�������������������1������ʯ����Ҫ�ɷ�Ϊ̼��ƣ��������ᷴӦ�����廯�ơ�ˮ�Ͷ�����̼�����ӷ���ʽΪCaCO3��2H��=Ca2����CO2����H2O����2��������ҵ����ʯ�ijɷּ�����ͼ֪�����ӵ�Ŀ���dz�ȥAl3����Fe3���������������Ϊ���������������ǿ��ǿ�������Һ��pHԼΪ8��0��Ŀ����ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣻��3����Һ�к������������ƣ����������ữ��Ŀ���dz�ȥ������Ca(OH)2����Һ�еþ���IJ���Ϊ����Ũ������ȴ�ᾧ�����˵ȣ���4�����������غ㶨��֪��n(CaBr2)= n(CaCO3)=0.02mol���廯�ƾ���(CaBr2��2H2O)������Ϊ4.72g,��������Ϊ4.72/5.00��100%=94��4%����5�����������ữ����������Һ���������ӣ�ʵ������Ϊ��������ɫ���ǣ����ӷ���ʽΪBr����Ag��=AgBr�������ò�������Һ��������ӣ�����Ϊ������ɫ���������ӷ���ʽΪCa2++C2O42-=CaC2O4����
���㣺������ѧ��������Ϊ���忼�����ӷ���ʽ����д����ѧʵ�������������ѧ���㼰���Ӽ��顣

�±����������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ����
| | NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 21.2 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |
�����裺�����ʱ��Ӱ����Ե��ܽ�ȣ����뾧��ʱ���ܼ�����ĺ��Բ��ƣ�
ijͬѧ��������ʵ���֮��Ϊ1��1�������ƺ��Ȼ���Ϊԭ�ϣ�����һ������ˮ��ȡ����ص�ʵ�飬����������ͼ��ʾ��

��1���ڢٺ͢ڵ�ʵ������У���Ҫ���ƵĹؼ���ʵ��������______________________�������������У�______���A����C����ӦΪ����ؾ��塣
��2���ڢٵ�ʵ������У���Ҫ���еIJ���������________________��________________��_____________��
��3���ֲ�Ʒ�п��ܺ�����������_______________________����������һ�����ӵķ�����________________________________________________________________________��
��4��Ϊ��ϴ�����õ�����ؾ��壬�����ܼ�������ϴ�Ӽ�����___________�����ţ���
a����ˮ b����ˮ c��95%�ľƾ� d�����Ȼ�̼
��5����ȡ34.0g�����ƺ�29.8g�Ȼ��أ�����70gˮ����100��������50gˮ��ά�ָ��¶ȣ����ˣ��������������Ϊ_______________��
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��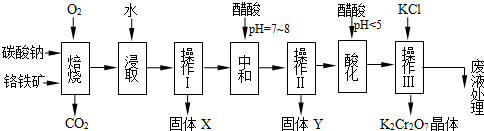
��֪����4FeO��Cr2O3+ 8Na2CO3+ 7O2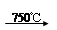 8Na2CrO4 + 2 Fe2O3 + 8CO2����
8Na2CrO4 + 2 Fe2O3 + 8CO2����
��Na2CO3 + Al2O3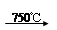 2NaAlO2 + CO2������ Cr2O72��+ H2O
2NaAlO2 + CO2������ Cr2O72��+ H2O 2CrO42�� + 2H+
2CrO42�� + 2H+
��1������X����Ҫ����_________����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��__________����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH��5����Ŀ����_________________________________��
| ���� | �ܽ��/(g/100gˮ) | ||
| 0��C | 40��C | 80��C | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� ��
���ˡ�_______�����
��4���ұ���������ʵ��ܽ�����ݣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl��K2Cr2O7��+2NaCl���÷�Ӧ����Һ���ܷ�����������_______________��
��5��������Һ�й���������ʹCr2O72-������ɫ�Ĺ���������CrO5���ӽṹΪ
 ), �÷�Ӧ����������Cr2O72-�Ĵ��ڡ�д����Ӧ�����ӷ���ʽ�� ��
), �÷�Ӧ����������Cr2O72-�Ĵ��ڡ�д����Ӧ�����ӷ���ʽ�� ���÷�Ӧ (����ڡ������ڡ�)������ԭ��Ӧ��
��6����ȡ�ظ��������2.500g���250mL��Һ,ȡ��25mL�����ƿ��,����10mL2mol/ L H2SO4�������⻯�أ����Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���I2+2S2O32����2I-+S4O62������
���жϴﵽ�ζ��յ�������� ��
����ʵ���й���ȥNa2S2O3����Һ40.00mL�������ò�Ʒ���ظ���صĴ���Ϊ ��������3λ��Ч����, K2Cr2O7��Ħ������Ϊ294g/mol����
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3·CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������С�
�ӷ����л��������ܣ�CoO���Ĺ����������£�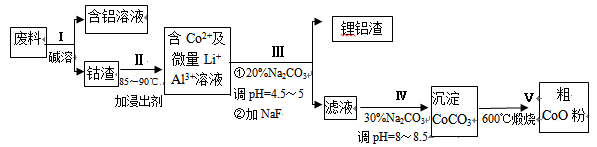
��1������I�в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ ��
��2������II�м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܡ�������ܵĻ�ѧ��Ӧ����ʽΪ��������ֻ��һ������� ����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ��ӷ�Ӧԭ������������������ܵ���Ҫԭ��_______________��
��3�����̢�õ����������Ҫ�ɷ���LiF��Al(OH)3��̼������Һ�ڲ���Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ________________________��
��4��̼������Һ�ڹ���III��IV����������������ͬ����д���ڹ���IV�����������
____________________________________________________________��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����______������ţ���
| A��c(Na+) = 2c(CO32-) | B��c(Na+) > c(CO32-) > c(HCO3-) |
| C��c(OH-) > c(HCO3-) > c(H+) | D��c(OH-) - c(H+)��c(HCO3-) + 2c(H2CO3) |
���й����ʵķ���ش��������⣺
��1������һƿA��B�Ļ��Һ����֪���ǵ��������±���
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
| A | ��11��5 | 198 | 1��11 | A��B���ܣ��Ҿ�������ˮ |
| B | 17��9 | 290 | 1��26 |
�ݴ˷�������A��B�����ij��÷����ǣ� ��
��2���ڷ�Һ©������һ���л��ܼ���ȡˮ��Һ���ij����ʱ�����÷ֲ�������֪����һ��Һ���ǡ�ˮ�㡱�������һ�ּ����жϷ���: ��

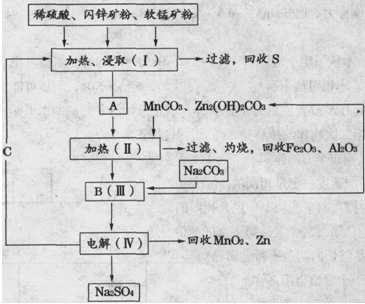
 MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��