��Ŀ����
ʵ��������������NH3�ڼ��ȵ���������CuO��ַ�Ӧ����Cu��N2��H2O�����ⶨCu�����ԭ��������װ��ͼ����
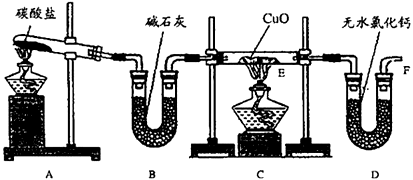
��1�����Ӻ�װ�ú����װ�������Եķ�����__________________��
��2����װ��A��B��ȡ���� ������İ��������Թ���̼���εĻ�ѧʽΪ___________��װ��B�м�ʯ�ҵ�������___________________��
��3��ʵ���й۲쵽Cװ�õ�E���в�����������_________________ ��E���з�����Ӧ�Ļ�ѧ����ʽΪ______________________��
��4����ʵ���в�����������ݣ� �ٿ�E�ܵ�����a�� ��ʵ��ǰE�ܺ�CuO��������b�� �۳�ַ�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����£������������� �ܳ�ַ�Ӧ��D�ܼ���ʢ���ʵ�������d ��ѡ������������г�����Cu�����ԭ�������ļ���ʽ����Cu�⣬�����漰����Ԫ�ص����ԭ��������Ϊ��֪����Ar(Cu)= __________________��
��2����װ��A��B��ȡ���� ������İ��������Թ���̼���εĻ�ѧʽΪ___________��װ��B�м�ʯ�ҵ�������___________________��
��3��ʵ���й۲쵽Cװ�õ�E���в�����������_________________ ��E���з�����Ӧ�Ļ�ѧ����ʽΪ______________________��
��4����ʵ���в�����������ݣ� �ٿ�E�ܵ�����a�� ��ʵ��ǰE�ܺ�CuO��������b�� �۳�ַ�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����£������������� �ܳ�ַ�Ӧ��D�ܼ���ʢ���ʵ�������d ��ѡ������������г�����Cu�����ԭ�������ļ���ʽ����Cu�⣬�����漰����Ԫ�ص����ԭ��������Ϊ��֪����Ar(Cu)= __________________��
��1����F�����ӵ��ܣ��������ܿڽ�û��ʢ��ˮ���ձ��У����Թ�A�����ܿ�������ð����ֹͣ���Ⱥ���ˮ�����뵼�����γ�һС��ˮ����˵��װ�õ����������á�
��2�� (NH4)2CO3��NH4HCO3 ������ˮ�ֺͶ�����̼
��3����ɫ�����ɺ�ɫ��������ˮ����֣�2NH3+ 3CuO 3Cu + N2+ 3H2O
3Cu + N2+ 3H2O
��4��
��2�� (NH4)2CO3��NH4HCO3 ������ˮ�ֺͶ�����̼
��3����ɫ�����ɺ�ɫ��������ˮ����֣�2NH3+ 3CuO
 3Cu + N2+ 3H2O
3Cu + N2+ 3H2O��4��


��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ