��Ŀ����
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����
CO(g)��2H2(g) CH3OH(g) ����������������
CH3OH(g) ����������������
CO(g)��2H2(g)
 CH3OH(g) ����������������
CH3OH(g) ����������������
��1���ϳɼ״���Ӧ�У��䷴Ӧ�ȡ�H__________0(�����������������)
��2���������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2 �����йظ���ϵ��˵����ȷ����______________
a�� ������Ũ�ȼ���
b�� ����Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c�� �״������ʵ�������
d�� ����ƽ��ʱn(H2)/n(CH3OH)����
��3�����о�����Ӧ������������õ�ΪCu2O����Ӧ��ϵ�к�����CO2������ά�ִ���Cu2O�������䣬ԭ����_____________________���û�ѧ����ʽ��ʾ����
��2���������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2 �����йظ���ϵ��˵����ȷ����______________
a�� ������Ũ�ȼ���
b�� ����Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c�� �״������ʵ�������
d�� ����ƽ��ʱn(H2)/n(CH3OH)����
��3�����о�����Ӧ������������õ�ΪCu2O����Ӧ��ϵ�к�����CO2������ά�ִ���Cu2O�������䣬ԭ����_____________________���û�ѧ����ʽ��ʾ����
��1����
��2��bc
��3��Cu2O+CO 2Cu+CO2
2Cu+CO2
��2��bc
��3��Cu2O+CO
 2Cu+CO2
2Cu+CO2 
��ϰ��ϵ�д�
�����Ŀ
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��CH3OH��g����������������и��⣺
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��CH3OH��g����������������и��⣺ һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g���������й�˵����ȷ���ǣ�������
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g���������й�˵����ȷ���ǣ�������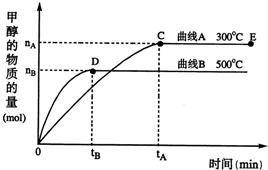 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO���� CH3OH��g��
CH3OH��g�� 2Cu+CO2
2Cu+CO2
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO������������������и��⣺
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO������������������и��⣺