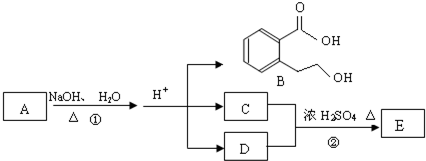
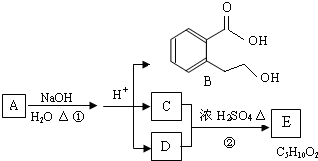
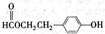��Ŀ����
A��B��C��D��E��Ϊ������ˮ�Ĺ��壬������ǵ�������
| ������ | NH4+��Ag+��Mg2+��Ba2+��Al3+ |
| ������ | Cl�D��OH�D��NO3�D��CO32�D |
�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
��A��Һ��B��C��D��Һ��Ӧ���վ����ɰ�ɫ������
��C��Һ��D��Һ��Ӧ���ɰ�ɫ������ͬʱ�ų���ɫ���壻
��D��Һ������E��Ӧ���ɰ�ɫ�������������E��Һ����ɫ������ʧ��
��1���ݴ��ƶ������ǣ��ѧʽ��
A ��B ��C ��D ��E ��
��2��д�����з�Ӧ�����ӷ��̣�
��C��Һ��E��Һ�ڼ��������·�Ӧ ��
�ڹ���E��Һ��D��Һ��Ӧ ��
��1��A��AgNO3 B��MgCl2 C����NH4��2CO3 D��AlCl3��E��Ba(OH)2
��2����2NH4++CO32�D++Ba2++2OH�D=BaCO3��+2NH3��+2H2O
��Al3++4OH�D=AlO2�D+2H2O

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ