��Ŀ����
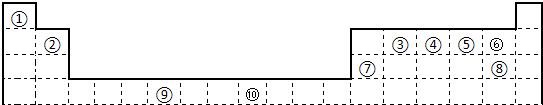
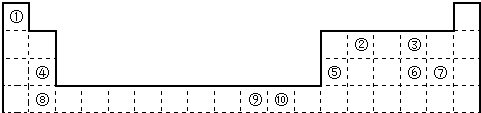
�±�Ϊ��ʽ���ڱ���һ���֣����е���Ŵ�����Ӧ��Ԫ�ء�

��1��д���ϱ���Ԫ�آ�ԭ�ӵ���Χ�����Ų�ʽ__________��
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ__________�ӻ���Ԫ�آ�����γɵĻ�����ľ���������__________��
��3��Ԫ�آܵĵ�һ������__________Ԫ�آݣ���д�������������������ĵ�һ�����ܣ�Ԫ�آ���Ԫ�آ��γɵ�X���ӵĿռ乹��Ϊ__________����д����Ԫ�آܵĵ��ʻ�Ϊ�ȵ�������ӡ����ӵĻ�ѧʽ__________����дһ�֣���
��4���ڲⶨԪ�آ�����γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ����__________��
��5��Ԫ�آܵ�����������Ӧ��ˮ����ϡ��Һ��Ԫ�آߵĵ��ʷ�Ӧʱ��Ԫ�آܱ���ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽΪ __________��
��6����������Xͨ�뺬��Ԫ�آ����ɫ��������Һ�У���Ӧ�����ӷ���ʽΪ__________��Ԫ�آ��ij��������ľ���ṹ��ͼ��ʾ������ʵ�����ʾԪ�آ�ԭ�ӣ���һ������������������ԭ����ĿΪ__________��
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ__________�ӻ���Ԫ�آ�����γɵĻ�����ľ���������__________��
��3��Ԫ�آܵĵ�һ������__________Ԫ�آݣ���д�������������������ĵ�һ�����ܣ�Ԫ�آ���Ԫ�آ��γɵ�X���ӵĿռ乹��Ϊ__________����д����Ԫ�آܵĵ��ʻ�Ϊ�ȵ�������ӡ����ӵĻ�ѧʽ__________����дһ�֣���
��4���ڲⶨԪ�آ�����γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ����__________��
��5��Ԫ�آܵ�����������Ӧ��ˮ����ϡ��Һ��Ԫ�آߵĵ��ʷ�Ӧʱ��Ԫ�آܱ���ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽΪ __________��
��6����������Xͨ�뺬��Ԫ�آ����ɫ��������Һ�У���Ӧ�����ӷ���ʽΪ__________��Ԫ�آ��ij��������ľ���ṹ��ͼ��ʾ������ʵ�����ʾԪ�آ�ԭ�ӣ���һ������������������ԭ����ĿΪ__________��

��1��3d54s1
��2��sp2�� ���Ӿ���
��3���� �� ������ CO�� C22-
��4��HF��̬ʱ���γɣ�HF��n���ӣ���HF���Ӽ���γ������
��5��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O
��6��Cu2+ +4NH3=[Cu��NH3��4]2+ �� Cu2+ +2OH-=Cu��OH��2�� ��Cu��OH��2 +4NH3=[Cu��NH3��4]2++OH- �� 2
��2��sp2�� ���Ӿ���
��3���� �� ������ CO�� C22-
��4��HF��̬ʱ���γɣ�HF��n���ӣ���HF���Ӽ���γ������
��5��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O
��6��Cu2+ +4NH3=[Cu��NH3��4]2+ �� Cu2+ +2OH-=Cu��OH��2�� ��Cu��OH��2 +4NH3=[Cu��NH3��4]2++OH- �� 2

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ