��Ŀ����
����ѧ����ѡ�ޣ����ʽṹ�����ʡ�( 15��)
ԭ�������������ӵ�A��B��C��D��E��F���ֳ���Ԫ���У�A��B��C��D�Ƕ����ڷǽ���Ԫ�أ�B��C��Dͬ���ڣ�E��F�ǵ������ڵĽ���Ԫ�أ�F���������ܲ����ȫ�������±�����Ҫ���ϼۼ�ԭ�Ӱ뾶���ݣ�
| | A | B | C | D | E | F |
| ��Ҫ���ϼ� | ��1 | ��3����5 | ��2 ��6 | ��1 ��7 | ��2 | ��1 ��2 |
| ԭ�Ӱ뾶nm | 0.071 | 0.11 | 0.102 | 0.099 | 0.197 | 0.117 |
��1��B��C��D����Ԫ�ص�һ��������ֵ��С�����˳���� (��Ԫ�ط���)��
��2��B���⻯������ԭ�Ӳ�ȡ �ӻ����ռ乹���� �Σ��� ����
(����ԡ��Ǽ��ԡ�)��
��3��F2����NH3 �γ������ӵĻ�ѧʽΪ ��F���ʾ��徧������ͼ��
(��١��ڡ��ۻ��)��
��4��A ��E����Ԫ���γɾ��徧������ͼ�е� (��١��ڡ��ۻ��)��A���ӵ���λ���� ��

��5����ͼ�������߷ֱ��ʾ��A�塢��A�塢��A�塢��A��Ԫ����̬�⻯��е�仯��
��ѡ��C���⻯�����ڵ����� (��n��m��x��y)��

��1��S��P��Cl ��2�֣�
��2��Sp3 ��1�֣� ������1�֣� ���ԣ�1�֣�
��3��[Cu(NH3)4]2����2�֣� �� ��2�֣�
��4���ڣ�2�֣� 4 ��2�֣�
��5�� n ����2�֣�
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����ѧ--ѡ��3���ʽṹ�����ʡ�
����ѧ--ѡ��3���ʽṹ�����ʡ�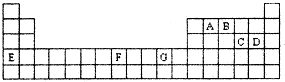
 ���������к��еĻ�ѧ��������
���������к��еĻ�ѧ��������


