��Ŀ����
�������ӷ���ʽ��ȷ����(����)
| A�����ô����ܽ⺬̼��Ƶ�ˮ����CaCO3��2H��=Ca2����H2O��CO2�� |
B����ͭΪ�缫��ⱥ��ʳ��ˮ��2Cl����2H2O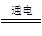 Cl2����H2����2OH�� Cl2����H2����2OH�� |
| C����������Һ�еμӹ�����ˮ��Al3����4NH3��H2O=AlO2����4NH4+��2H2O |
| D����С�մ���Һ�м�����Ba(OH)2��Һ��2HCO3����Ba2����2OH��=BaCO3����2H2O��CO32�� |
D
����

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
�ڳ����£�0.1000mol��L-1Na2CO3��Һ25mL ��0.1000mol��L-1����ζ�����ζ�������ͼ���Եζ�������������Һ���������Ũ�ȼ�Ĺ�ϵ�������й�˵����ȷ���ǣ� ��
| A��a�㣺c��CO32-��=c(HCO3-)>c(OH-) |
| B��b�㣺5c(Cl-)=4c(HCO3-)+4c(CO32-) |
| C��c�㣺c(OH-)+ c(CO32-)=c(H+)+ c(H2CO3) |
| D��d�㣺c(Na+)<c(Cl-) |
�������ӷ���ʽ��ȷ����
A����H2C2O4�м�������KMnO4��Һ�� |
B��Ca(HCO3)2�����Ca(OH)2��Һ��Ӧ�� |
C���ö��Ե缫�������ͭ��Һ�� |
D������̼��������Һ������������Һ��ϣ� |
ij��ɫ��Һ�к���K+��Cl-��OH-��S ��S
��S ,Ϊ�˼����OH-�����������������,�������ᡢ���ᡢ��������Һ������������Һ����ˮ�ͷ�̪�����Լ�,�������ʵ�鲽��,����¼�������:
,Ϊ�˼����OH-�����������������,�������ᡢ���ᡢ��������Һ������������Һ����ˮ�ͷ�̪�����Լ�,�������ʵ�鲽��,����¼�������: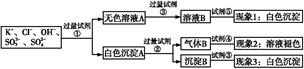
�����йؽ��۴������(����)
| A���Լ�����AgNO3��Һ,�Լ�����HNO3,����1�а�ɫ������AgCl |
| B������3�а�ɫ������BaSO4 |
| C���Լ���������,�Լ��������� |
D����������2�����ӷ���ʽ��:Br2+2H2O+SO2 4H++2Br-+S 4H++2Br-+S |
����˵����ȷ���ǣ� ��
A��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ:4Mg+6H++N =4Mg2++N =4Mg2++N +3H2O +3H2O |
| B��������,0.1 mol/LһԪ��HA��Һ��c(OH-)/c(H+)=1��10-8,�����Һ��pH=3 |
| C����ͬ������,Ũ�Ⱦ�Ϊ0.01 mol/L��NH4Cl��Һ��NaCl��Һ��,ǰ�ߵ���������Ũ�ȴ��ں��ߵ���������Ũ�� |
| D�����ʵ���Ũ����ȵĴ��������������Һ�������Ϻ����Һ��:c(Na+)+c(H+)=c(CH3COO-)+c(OH-)+c(CH3COOH) |
�ס�������Һ��,�ֱ��д�����Cu2+��K+��H+��Cl-��C ��OH- 6�������е�3��,��֪����Һ����ɫ,������Һ�д������ڵ������ǣ� ��
��OH- 6�������е�3��,��֪����Һ����ɫ,������Һ�д������ڵ������ǣ� ��
A��K+��OH-��C | B��Cu2+��H+��Cl- |
| C��K+��H+��Cl- | D��C ��OH-��Cl- ��OH-��Cl- |
�����йػ�ѧ�����ʹ����ȷ���ǣ�������
| A��Cl2ͨ��ʯ��������Ư�۵����ӷ���ʽ�� Cl2��2OH��=Cl����ClO����H2O |
| B��������ĭ��������ʱ�����ķ�Ӧ��2Al3����CO32-��3H2O=2Al��OH��3����CO2�� |
| C����֪���ӵĻ�ԭ�ԣ�Sn2����Fe2��������Ʋ�����Һ���ܷ������·�Ӧ��Sn4����2Fe2��=2Fe3����Sn2�� |
D��298 Kʱ����֪12 gʯī��ȫȼ������CO2��g���ų�393.5 kJ������1 mol CO��ȫȼ�շų�283.5 kJ��������һ���У�C��s��ʯī����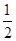 O2��g��=CO��g�� ��H����110 kJ��mol��1 O2��g��=CO��g�� ��H����110 kJ��mol��1 |

