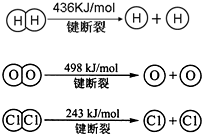
��Ŀ����
�ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g�����ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϣ���Ҫ��ش��������⣺
��1���ϳɰ���Ӧ�������仯��ͼ��ʾ����÷�Ӧ���Ȼ�ѧ��ʽΪ�� ��
��2�����¶Ⱥ㶨Ϊ298K������㶨Ϊ10L���ܱ������м������������������ʵ����ֱ�Ϊ0.7mol��0.6mol��ƽ��ʱ��õ�����ת����Ϊ
 ������¶�������÷�Ӧ��ƽ�ⳣ��K= ��
������¶�������÷�Ӧ��ƽ�ⳣ��K= ����3������£�����22.4mL�İ���ͨ��100mLpHΪ2�������У�����Һ�и�����Ũ�ȵ�˳���ɴ�СΪ ��
��4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4���ù��̵Ļ�ѧ����ʽΪ ���ɴ˿�֪Ksp��CaSO4�� Ksp��CaCO3��������ڡ�����С�ڡ����ڡ���

���𰸡���������1������ͼ������жϣ���Ӧ�ʱ�=���ѻ�ѧ����������-��Ӧ���γɻ�ѧ���ų�����=508-600=-92KJ/mol�������Ȼ�ѧ����ʽ��д����ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ�ʱ䣻
��2�����ݻ�ѧƽ������ʽ��ʽ����ƽ��Ũ�ȣ����ƽ�ⳣ���������õ���
��3������1molNH3��10-3molHCl��Ӧ����NH4Cl���������������ж���Һ������Ũ�ȣ�
��4�����ݽ�����ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4����Ӧ����������狀�̼�����ƣ���ƽ��д��ѧ����ʽ�����ݳ���ת�������ж��ܶȻ�������С����������Һ���������̼��Ӧ����̼��泥����˿ɵã�NH4��2SO4������ΪCaCO3��˵��CaCO3�����ܣ�CaSO4��CaCO3�Ĺ���������ͬΪAB�ͣ���ͬ�����ܽ��ԽС���ܶȻ�ԽС����
����⣺��1�����ͼ�������֪��Ӧ���ʱ�Ϊ-92KJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92KJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92KJ/mol��
��2�����ݻ�ѧƽ������ʽ��ʽ���㣬����㶨Ϊ10L���ܱ�������ƽ��ʱ��õ�����ת����Ϊ ��
��
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol�� 0.6 0.7 0
ת������mol�� 0.6× 0.6 0.4
0.6 0.4
ƽ������mol�� 0.4 0.1 0.4
K= =
= =4×104��
=4×104��
�ʴ�Ϊ��4×104��
��3��1molNH3��10-3molHCl��Ӧ����NH4Cl����Һ���Ȼ�����ʵ���Ϊ10-3mol��һˮ�ϰ����ʵ���1-0.001=0.999mol��������Һ������Ũ�ȴ�СΪ��c��NH4+����c��OH-����c��Cl-����c��H+����
�ʴ�Ϊ��c��NH4+����c��OH-����c��Cl-����c��H+����
��4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4������ΪCaCO3��˵��CaCO3�����ܣ�CaSO4��CaCO3�Ĺ���������ͬΪAB�ͣ���ͬ�����ܽ��ԽС���ܶȻ�ԽС����Ksp��CaSO4����Ksp��CaCO3�����ù��̵Ļ�ѧ����ʽΪ��CaSO4+2NH3+CO2+H2O=CaCO3��+��NH4��2SO4��
�ʴ�Ϊ��CaSO4+2NH3+CO2+H2O=CaCO3��+��NH4��2SO4������
���������⿼���Ȼ�ѧ����ʽ��д��������ѧƽ��ļ���Ӧ�ã�ƽ�ⳣ��������㣬��Һ������Ũ�ȴ�С�Ƚϣ�����ת��ԭ���ķ���Ӧ�ã���Ŀ�Ѷ��еȣ�
��2�����ݻ�ѧƽ������ʽ��ʽ����ƽ��Ũ�ȣ����ƽ�ⳣ���������õ���
��3������1molNH3��10-3molHCl��Ӧ����NH4Cl���������������ж���Һ������Ũ�ȣ�
��4�����ݽ�����ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4����Ӧ����������狀�̼�����ƣ���ƽ��д��ѧ����ʽ�����ݳ���ת�������ж��ܶȻ�������С����������Һ���������̼��Ӧ����̼��泥����˿ɵã�NH4��2SO4������ΪCaCO3��˵��CaCO3�����ܣ�CaSO4��CaCO3�Ĺ���������ͬΪAB�ͣ���ͬ�����ܽ��ԽС���ܶȻ�ԽС����
����⣺��1�����ͼ�������֪��Ӧ���ʱ�Ϊ-92KJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92KJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92KJ/mol��
��2�����ݻ�ѧƽ������ʽ��ʽ���㣬����㶨Ϊ10L���ܱ�������ƽ��ʱ��õ�����ת����Ϊ
 ��
��N2��g��+3H2��g��?2NH3��g��
��ʼ����mol�� 0.6 0.7 0
ת������mol�� 0.6×
 0.6 0.4
0.6 0.4ƽ������mol�� 0.4 0.1 0.4
K=
 =
= =4×104��
=4×104���ʴ�Ϊ��4×104��
��3��1molNH3��10-3molHCl��Ӧ����NH4Cl����Һ���Ȼ�����ʵ���Ϊ10-3mol��һˮ�ϰ����ʵ���1-0.001=0.999mol��������Һ������Ũ�ȴ�СΪ��c��NH4+����c��OH-����c��Cl-����c��H+����
�ʴ�Ϊ��c��NH4+����c��OH-����c��Cl-����c��H+����
��4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4������ΪCaCO3��˵��CaCO3�����ܣ�CaSO4��CaCO3�Ĺ���������ͬΪAB�ͣ���ͬ�����ܽ��ԽС���ܶȻ�ԽС����Ksp��CaSO4����Ksp��CaCO3�����ù��̵Ļ�ѧ����ʽΪ��CaSO4+2NH3+CO2+H2O=CaCO3��+��NH4��2SO4��
�ʴ�Ϊ��CaSO4+2NH3+CO2+H2O=CaCO3��+��NH4��2SO4������
���������⿼���Ȼ�ѧ����ʽ��д��������ѧƽ��ļ���Ӧ�ã�ƽ�ⳣ��������㣬��Һ������Ũ�ȴ�С�Ƚϣ�����ת��ԭ���ķ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
�����Ŀ
 �ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1���ڷ�Ӧ�����У�����Ӧ���ʵı仯��ͼ������˵����ȷ���ǣ�������
�ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1���ڷ�Ӧ�����У�����Ӧ���ʵı仯��ͼ������˵����ȷ���ǣ������� �ϳɰ���ũҵ���������������������Ҫ���壮
�ϳɰ���ũҵ���������������������Ҫ���壮