��Ŀ����
|
����(H2)��һ����̼(CO)������(C8H18)������(CH4)���Ȼ�ѧ����ʽ�ֱ�Ϊ�� H2(g)��1/2O2(g)��H2O(l)����H����285.8 kJ/mol��CO(g)��1/2O2(g) ��CO2(g)����H����283.0 kJ/mol��C8H18(l)��25/2O2(g)��8CO2(g)��9H2O(l)����H����5518 kJ/mol��CH4(g)��2O2(g)��CO2(g)��2H2O(l)����H����890.3 kJ/mol ��ͬ������ H2��CO��C8H18��CH4��ȫȼ��ʱ���ų��������ٵ��� | |
| [����] | |
A�� |
H2(g) |
B�� |
C8H18(l) |
C�� |
CO(g) |
D�� |
CH4(g) |
�𰸣�C

��ϰ��ϵ�д�
�����Ŀ
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺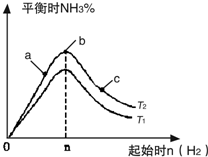 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺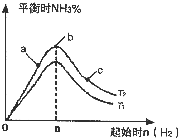 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺