��Ŀ����
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ���λ��Ϊ6�������F����������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ________��
(2)B���⻯��ķ��ӿռ乹����________��������ԭ�Ӳ�ȡ________�ӻ���
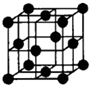
(3)д��������AC2�Ľṹʽ________��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ________��
(4)E�ĺ�������Ų�ʽ��________�������F�Ļ�ѧʽΪ________��
(5)B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��________��
(6)B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߵ�ԭ���ǣ�________��
������
|
����(1)C��O��N(1��) ����(2)������(1��)��sp3(1��) ����(3)O��C��O(1��)��N2O(1��) ����(4)1s22s22p63s23p63d54s1(��[Ar]3d54s1)(1��)��[Cr(NH3)4(H2O)2]Cl3(1��) ����(5)4Mg��10HNO3��4Mg(NO3)2��NH4NO3��3H2O(2��) ����(6)NH3��H2O���Ӽ���������ʹ�����۷е������ˣ�(2��) |

| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�