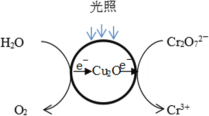
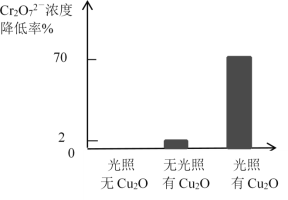
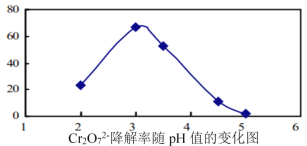
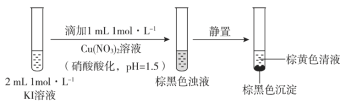
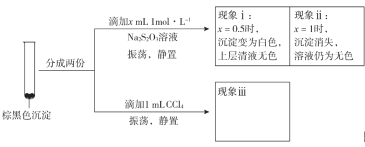
��Ŀ����
����Ŀ�����ж���������Ԫ��X��Y��Z��R��T��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵ�Z2T�ͻ��������ƻ�ˮ�ĵ���ƽ�⣬����Ԫ�ص�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������
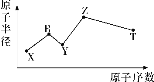
A.ԭ�Ӱ뾶�����Ӱ뾶�����㣺Y��Z
B.���⻯��ķе�����ȶ��Ծ����㣺Y>T
C.����������Ӧ��ˮ��������ԣ�T��R
D.�����£�0.1mol��L��1��X��Y��Z��T����Ԫ����ɵĻ������ˮ��Һ��pHһ������1
���𰸡�B
��������
Rԭ�������������ǵ��Ӳ�����2������R����ΪC��S�������ͼ��֪RΪC��Y��Z���γ�Z2Y��Z2Y2�����ӻ������YΪO��ZΪNa��Z��T�γɵ�Z2T�ͻ��������ƻ�ˮ�ĵ���ƽ�⣬��TΪS��X��ԭ�Ӱ뾶��ԭ��������С����XΪH��
A. ���Ӳ�Խ�࣬ԭ�Ӱ뾶Խ����ԭ�Ӱ뾶ΪO��Na��������ͬ�����Ų��������У�ԭ������������Ӱ뾶С�������Ӱ뾶ΪO2����Na������A����
B. Y��T�ļ��⻯��ֱ�ΪH2O��H2S��ˮ���Ӽ�����������е�H2O>H2S��Ԫ�طǽ�����Խǿ����Ӧ�ļ��⻯������ȶ���Խǿ�������ȶ���H2O>H2S����B��ȷ��
C. �ǽ�����S��C��������������Ӧ��ˮ���������ΪH2SO4��H2CO3����C����
D. ��H��O��Na��S����Ԫ����ɵij�����������NaHSO3��NaHSO4��0.1mol��L��1NaHSO3��Һ��pH>1��0.1mol��L��1NaHSO4��Һ��pH��1����D����
������������ΪB��

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�