��Ŀ����
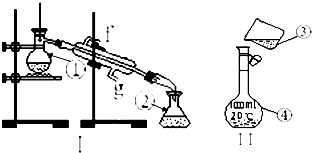
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
��1��д���������������ƣ���______����______��
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ����______��������ţ�
��3��������װ��I��ȡ����ˮ����ȱ�ٵ�������______��������������������е������������Ϊ��______����ȴˮ��______�ڽ���
��4����������250mL 0.2mol?L-1 NaCl��Һ������װ��II��ijͬѧ���ƴ���Һʱת�Ʋ�����ʾ��ͼ��ͼ������������ֱ���______��______��
��1��д���������������ƣ���______����______��
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ����______��������ţ�
��3��������װ��I��ȡ����ˮ����ȱ�ٵ�������______��������������������е������������Ϊ��______����ȴˮ��______�ڽ���
��4����������250mL 0.2mol?L-1 NaCl��Һ������װ��II��ijͬѧ���ƴ���Һʱת�Ʋ�����ʾ��ͼ��ͼ������������ֱ���______��______��

��1������װ���е���Ҫ������������ƿ�������ܡ�ţ�ǹܡ���ƿ���ƾ��ƣ���Ϊ������ƿ��Ϊ�����ܣ��ʴ�Ϊ��������ƿ�������ܣ�
��2������ƿ��ʹ��ǰһ��Ҫ��©���ʴ�Ϊ���ܣ�
��3����ȡ����ˮ��ʵ����������̣������þƾ��ƣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ�����g��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ���ʴ�Ϊ��δ�ò�����������δ����250ml����ƿ��
��2������ƿ��ʹ��ǰһ��Ҫ��©���ʴ�Ϊ���ܣ�
��3����ȡ����ˮ��ʵ����������̣������þƾ��ƣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ�����g��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ���ʴ�Ϊ��δ�ò�����������δ����250ml����ƿ��

��ϰ��ϵ�д�
�����Ŀ
 �������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
 ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã� �������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�