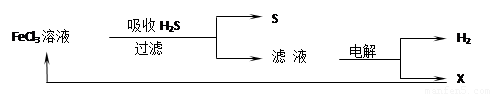��Ŀ����
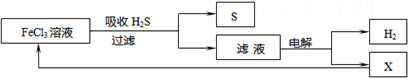
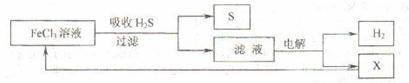
��ͼ��ij�о�С����õ�ⷨ����ʯ�����ƹ����в����Ĵ���H2S�����Ĺ������̣��÷�����H2S�������ʴ�99%���ϣ�������ȡH2��S������˵����ȷ���ǣ�������
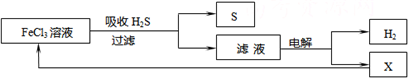

| A������H2S�����ӷ���ʽΪ��2Fe3++H2S=2Fe2++S��+2H+ |
| B���������е�������Ӧ��ҪΪ��2Cl--2e-=Cl2�� |
| C���ù�������������ɫ��ѧ˼�� |
| D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ |
A����ͼ�е�����ͼ��֪��FeCl3��H2S��Ӧ����S��Fe2+�����ӷ���ʽ��ȷ����ʧ���������غ�ģ���A��ȷ��
B���������Ȼ�������������Һ������������Ӧ����������Ӧ��������Һ�к���Fe2+��H+��Cl-�����ڻ�ԭ��Fe2+��Cl-�������ͼʾѭ���������ʱ������ӦʽΪFe2+-e-�TFe3+����B����
C���ù��������յ�H2S���ת��ΪH2��S�����������ɫ��ѧ����C��ȷ��
D������H2S���ȼ�յĻ�ѧ����ʽΪ2H2S+3O2�T2SO2+2H2O�����ɵ�SO2Ҳ��һ���ж����壬��D����
��ѡ��A��C
B���������Ȼ�������������Һ������������Ӧ����������Ӧ��������Һ�к���Fe2+��H+��Cl-�����ڻ�ԭ��Fe2+��Cl-�������ͼʾѭ���������ʱ������ӦʽΪFe2+-e-�TFe3+����B����
C���ù��������յ�H2S���ת��ΪH2��S�����������ɫ��ѧ����C��ȷ��
D������H2S���ȼ�յĻ�ѧ����ʽΪ2H2S+3O2�T2SO2+2H2O�����ɵ�SO2Ҳ��һ���ж����壬��D����
��ѡ��A��C

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
��ͼ��ij�о�С����õ�ⷨ����ʯ�����ƹ����в����Ĵ���H2S�����Ĺ������̣��÷�����H2S�������ʴ�99%���ϣ�������ȡH2��S������˵����ȷ���ǣ�������
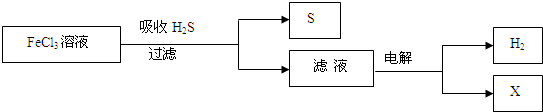

| A������H2S�����ӷ���ʽΪ��2Fe3++H2S�T2Fe2++S��+2H+ | B���������е�������Ӧ��ҪΪ��2Cl--2e-�TCl2�� | C��������X�ڸù��������п�ѭ������ | D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ |