��Ŀ����
���������μ����ֽ��Ҳ���ϸ��ӡ�ijѧϰС����Mg(NO3)2Ϊ�о�������ͨ��ʵ��̽�����ȷֽ�IJ���������4�ֲ��룺
�ף�Mg(NO3)2��NO2��O2 �ң�MgO��NO2��O2 ����Mg3N2��O2 ����MgO��NO2��N2
��1��ʵ��ǰ��С���Ա�������϶����붡��������������____ ________��
�������ϵ�֪��2NO2+2NaOH=NaNO3+NaNO2+H2O
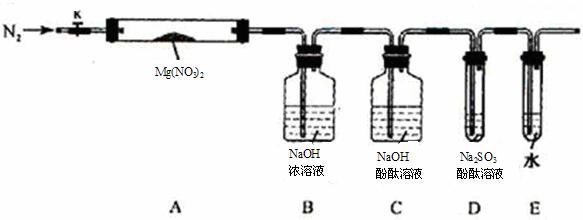
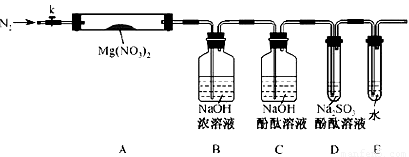
��Լס��ҡ������룬�������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
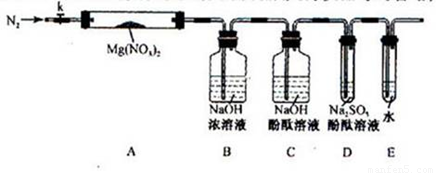
��2��ʵ�����
��ȡ�����Ӻ��˹����Լ�֮ǰ���ر�k����Ӳ�ʲ����ܣ�A�����۲쵽E �������������ų�������_ _______
�� ��ȡMg(NO3)2����3 . 79 g����A�У�����ǰͨ��N2������װ���ڵĿ�������Ŀ����________���ر�K���þƾ��Ƽ���ʱ����ȷ��������________Ȼ��̶��ڹ��й��岿λ�¼��ȡ�
�� �۲쵽A ���к���ɫ������֣�C��D ��δ�����Ա仯��
�� ����Ʒ��ȫ�ֽ⣬A װ����ȴ�����¡����������ʣ����������Ϊ1.0g
�� ȡ����ʣ��������Թ��У���������ˮ��δ����������
��3��ʵ������������
�� ����ʵ�������ʣ�����������������ɳ���ȷ�ϲ���_______����ȷ�ġ�
�� ����D ������������һλͬѧ��Ϊ����ȷ�Ϸֽ��������O2����Ϊ����O2��D�н�����������ԭ��Ӧ��_________________ ____����д��ѧ����ʽ������Һ��ɫ����ȥ��С�������϶��ֽ��������O2���ڣ�δ��ൽ��ԭ����_____________________��
�� С�����ۺ��ɵĹ�ʶ������ʵ������Բ����ƣ���Ľ�װ���һ���о���
��1��������������ԭ��Ӧԭ����2�֣�
��2����װ�����������ã�2�֣�
�ڱ���Բ���O2�ļ���������ţ�2�֣����ƶ��ƾ���Ԥ��Ӳ�ʲ����ܣ�2�֣�
��3������ ��2�֣� ��2Na2SO3 + O2 = 2Na2SO4��2�֣�
O2��ͨ��װ��Bʱ�Ѳ��뷴Ӧ��2�֣�
��������
������������⿼��Mg(NO)3���ȷֽ��IJ����̽�����Լ�ʵ�����������
��1��Mg(NO3)2��Mg��N��O�Ļ��ϼ۷ֱ�Ϊ��2����5����2�ۣ�NO2��N2��Mg2N3�е��Ļ��ϼ۷ֱ�Ϊ��4��0����3�ۣ�O2�����Ļ��ϼ���0�ۡ��ʼס��ҡ�����Ԫ�صĻ��ϼۡ������н���������������ԭ��Ӧ�ص㡣��������ֻ�л��ϼ۵Ľ��ͣ�û�л��ϼ۵����ߣ��ò²ⲻ������
��2�����һ��װ���������ݣ�˵������װ�����������á�N2��װ���еĿ����ϳ���Ŀ�ģ��Ƿ�ֹ�Էֽ�����O2�ļ��鳬�ɸ��š����Ȳ�������Ԥ�ȣ��ټ��м��ȡ�
��3����Mg(NO3)2������ˮ��Mg3N2�ܹ���ˮ��Ӧ��Mg3N2��6H2O��3Mg(OH)2����2NH3����MgO������ˮ������������ˮ��δ�����������Ҳ�����ȷ��
��Na2SO3Ϊ����ǿ���Σ�SO32-ˮ����Һ�ʼ��ԣ�SO32-��H2O HSO3-��OH-����Һ��ɫ��ȥ��˵�������ܹ�����Na2SO3ΪNa2SO4��2Na2SO3��O2��2Na2SO4��
HSO3-��OH-����Һ��ɫ��ȥ��˵�������ܹ�����Na2SO3ΪNa2SO4��2Na2SO3��O2��2Na2SO4��
δ���鵽��������������NO2��O2��ˮ��ͬ���ÿ��Բ���HNO3����NaOH���գ�����������O2�Ĵ��ڡ�
���㣺���黯ѧʵ������������

 ��У����ϵ�д�
��У����ϵ�д�
 2KNO2��O2��
2KNO2��O2�� 2CuO��4NO2����O2��
2CuO��4NO2����O2�� 2Ag��2NO2����O2��
2Ag��2NO2����O2��
