��Ŀ����
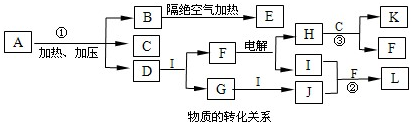
�л�������A��G������ת����ϵ��

��֪��

��B2H6Ϊ�����飩
����д���пհף�
��1��11.2L����״��������A�������г��ȼ�տ��Բ���88gCO2��45gH2O��A�ķ���ʽ�ǣ�______��
��2��B��C��Ϊһ�ȴ��������ǵĹ�ϵ��______��
��3����Ӧ�ٵķ�Ӧ�����ǣ�______��
��4����Ӧ�ڵĻ�ѧ����ʽ�ǣ�______��
��5��������ͬ�����ŵ�F��ͬ���칹��Ľṹʽ�ǣ�______��
��6����һ�������£���D�ۺϵõ��ĸ߷��ӻ�����Ľṹ��ʽ�ǣ�______��

��֪��

��B2H6Ϊ�����飩
����д���пհף�
��1��11.2L����״��������A�������г��ȼ�տ��Բ���88gCO2��45gH2O��A�ķ���ʽ�ǣ�______��
��2��B��C��Ϊһ�ȴ��������ǵĹ�ϵ��______��
��3����Ӧ�ٵķ�Ӧ�����ǣ�______��
��4����Ӧ�ڵĻ�ѧ����ʽ�ǣ�______��
��5��������ͬ�����ŵ�F��ͬ���칹��Ľṹʽ�ǣ�______��
��6����һ�������£���D�ۺϵõ��ĸ߷��ӻ�����Ľṹ��ʽ�ǣ�______��
��1����D�Ľṹ��֪��A�к���4��Cԭ�ӣ�Ϊ���壬11.2L����״��������A���ʵ���Ϊ0.5mol���������г��ȼ�տ��Բ���88gCO2�����ʵ���Ϊ2mol������45gH2O�����ʵ���Ϊ
=2.5mol����Hԭ����ĿΪ
=10����AΪC4H10���ʴ�Ϊ��C4H10��
��2��B��C��Ϊ�춡���һ�ȴ���������Ϊͬ���칹�壬�ʴ�Ϊ��ͬ���칹�壻
��3����Ӧ���ǣ�CH3��2CHCH2OH�����Ը�������������ɣ�CH3��2CHCOOH���ʴ�Ϊ��������Ӧ��
��4���ɷ�Ӧ��Ϣ��֪��EΪ��CH3��2CHCH2OH��E�����Ը��������������F��FΪ��CH3��2CHCOOH��E��F����������Ӧ����GΪ��CH3��2CHCOOCH2CH��CH3��2����Ӧ����ʽΪ
��CH3��2CHCH2OH+��CH3��2CHCOOH
��CH3��2CHCOOCH2CH��CH3��2+H2O��
�ʴ�Ϊ����CH3��2CHCH2OH+��CH3��2CHCOOH
��CH3��2CHCOOCH2CH��CH3��2+H2O��
��5���루CH3��2CHCOOH������ͬ�Ĺ����ţ���F�Ľṹ��ʽ��֪������ΪCH3CH2CH2-����ͬ���칹��ΪCH3CH2CH2COOH���ʴ�Ϊ��CH3CH2CH2COOH��
��6����CH3��2C=CH2ͨ���Ӿ۷�Ӧ���ɸ߾���

���ʴ�Ϊ��

��
| 45g |
| 18g/mol |
| 2.5mol��2 |
| 0.5mol |
��2��B��C��Ϊ�춡���һ�ȴ���������Ϊͬ���칹�壬�ʴ�Ϊ��ͬ���칹�壻
��3����Ӧ���ǣ�CH3��2CHCH2OH�����Ը�������������ɣ�CH3��2CHCOOH���ʴ�Ϊ��������Ӧ��
��4���ɷ�Ӧ��Ϣ��֪��EΪ��CH3��2CHCH2OH��E�����Ը��������������F��FΪ��CH3��2CHCOOH��E��F����������Ӧ����GΪ��CH3��2CHCOOCH2CH��CH3��2����Ӧ����ʽΪ
��CH3��2CHCH2OH+��CH3��2CHCOOH
| Ũ���� |
| �� |
�ʴ�Ϊ����CH3��2CHCH2OH+��CH3��2CHCOOH
| Ũ���� |
| �� |
��5���루CH3��2CHCOOH������ͬ�Ĺ����ţ���F�Ľṹ��ʽ��֪������ΪCH3CH2CH2-����ͬ���칹��ΪCH3CH2CH2COOH���ʴ�Ϊ��CH3CH2CH2COOH��
��6����CH3��2C=CH2ͨ���Ӿ۷�Ӧ���ɸ߾���

���ʴ�Ϊ��

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 NH4++NH2-
NH4++NH2-


