��Ŀ����
��11�֣�(1)������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㡣
��֪�� CH4(g)��H2O(g)��CO(g)��3H2(g) ��H��+206.2kJ��mol��1
CH4(g)��CO2(g)��2CO(g)��2H2(g) ��H��+247.4 kJ��mol��1
(1)�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ______________________________;
(2)�����������ܱ������н��У�1����Ӧ���ﵽƽ���������ƶ������������ƶ���������������С�����䡱����
���������������ƽ��___________��c(H2)____________(��ԭƽ�����);
�������¶ȣ�����Ӧ����________ ���淴Ӧ����___________;
��ϵ��ѹǿ__________��ƽ��___________��ƽ�ⳣ��_____________;
�ۼ��������H2�����ʵ���___________________��
��11�֣�(1)CH4(g) + 2H2O (g) = CO2(g) + 4H2(g) ��H=+165.0kJ��mol��1
(2)�����ƶ�����С����������;���������ƶ������� �۲��䡣
����������1�������˹���ɵ�Ӧ�á�������֪��Ӧ��֪�١�2���ڼ��õ�CH4(g) + 2H2O (g) = CO2(g) + 4H2(g) �����Է�Ӧ����+206.2kJ��mol��1��2��247.4 kJ��mol��1����165.0kJ��mol��1��
��2����Ӧ���������ġ����ȵĿ��淴Ӧ�����������ݻ���ѹǿ���ͣ�ƽ�������ƶ�����������Ũ����Ȼ�Ǽ�С�ģ������¶ȣ���Ӧ���ʶ�������ģ�ƽ�������ƶ�������ƽ�ⳣ�������¶����ߣ�ѹǿ������ֻ�ܸı䷴Ӧ���ʣ�������Ӱ��ƽ��״̬���������������ʵ������䡣

 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮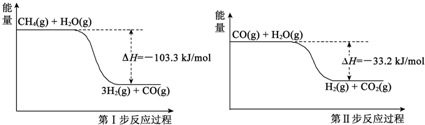 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮