��Ŀ����
����Ϣʹ��ij������ΪX,��ҵ�ϳɹ������£�
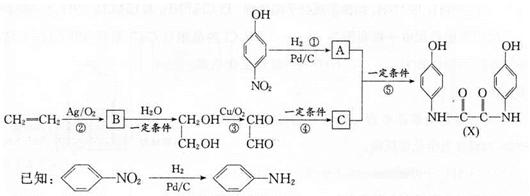
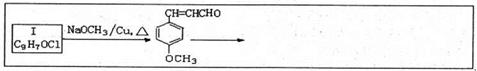
��1��A�й����ŵ�����Ϊ______;�����ٵ��ݵķ�Ӧ������������Ӧ����______ (�����)��
��2��д����ѧ����ʽ:��Ӧ��_______;��Ӧ��______��
��3��������������������Ϣʹ��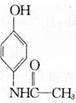 ��ͬ���칹��Ľṹ��ʽΪ______(д����)��
��ͬ���칹��Ľṹ��ʽΪ______(д����)��
�ٱ����ɲ���2�ֺ˴Ź����������շ�
�ڱ�����ȡ����һ����C����N,������N����C
������ͼ���¾�����ˮ��
��4��������������ֺϳ� �ķ������£�
�ķ������£�
����һ��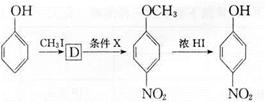
��������
������XΪ______;�뷽��һ��ȣ������������У�ԭ����______��
�����������һ�ĺϳɷ���,��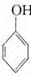 ��
�� Ϊԭ�ϣ����Լ���ѡ�������ٲ���ϳ�
Ϊԭ�ϣ����Լ���ѡ�������ٲ���ϳ�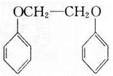 ��д���ϳ�·��(��ʾ�����緽��һ��: ____________��
��д���ϳ�·��(��ʾ�����緽��һ��: ____________��

��1��A�й����ŵ�����Ϊ______;�����ٵ��ݵķ�Ӧ������������Ӧ����______ (�����)��
��2��д����ѧ����ʽ:��Ӧ��_______;��Ӧ��______��
��3��������������������Ϣʹ��
 ��ͬ���칹��Ľṹ��ʽΪ______(д����)��
��ͬ���칹��Ľṹ��ʽΪ______(д����)���ٱ����ɲ���2�ֺ˴Ź����������շ�
�ڱ�����ȡ����һ����C����N,������N����C
������ͼ���¾�����ˮ��
��4��������������ֺϳ�
 �ķ������£�
�ķ������£�����һ��

��������

������XΪ______;�뷽��һ��ȣ������������У�ԭ����______��
�����������һ�ĺϳɷ���,��
 ��
�� Ϊԭ�ϣ����Լ���ѡ�������ٲ���ϳ�
Ϊԭ�ϣ����Լ���ѡ�������ٲ���ϳ� ��д���ϳ�·��(��ʾ�����緽��һ��: ____________��
��д���ϳ�·��(��ʾ�����緽��һ��: ____________����16�֣���1�����������ǻ���2�֣��ڢۢܣ�2�֣��ٴ��1�֣���ȫ�Կ�1�֣�
��2��HOCH2CH2OH+O2 OHCCHO+2H2O��2�֣�����ΪAgҲ���֣�
OHCCHO+2H2O��2�֣�����ΪAgҲ���֣�
2 +HOOCCOOH
+HOOCCOOH
 +2H2O��2�֣�ûд������1�֣�
+2H2O��2�֣�ûд������1�֣�
��3�� ��
�� ��
�� ��2�֣���1�֣�
��2�֣���1�֣�
��4����Ũ���� ���ȣ�2�֣���1�֣�д��ŨH2SO4��ŨHNO3������Ҳ���֣������ױ�������2�֣�
��CH2=CH2 BrCH2CH2Br
BrCH2CH2Br
 ��2�֣�д��һ�������1�֣�����±�ص���Ҳ���ԣ�
��2�֣�д��һ�������1�֣�����±�ص���Ҳ���ԣ�
��2��HOCH2CH2OH+O2
 OHCCHO+2H2O��2�֣�����ΪAgҲ���֣�
OHCCHO+2H2O��2�֣�����ΪAgҲ���֣�2
 +HOOCCOOH
+HOOCCOOH
 +2H2O��2�֣�ûд������1�֣�
+2H2O��2�֣�ûд������1�֣���3��
 ��
�� ��
�� ��2�֣���1�֣�
��2�֣���1�֣���4����Ũ���� ���ȣ�2�֣���1�֣�д��ŨH2SO4��ŨHNO3������Ҳ���֣������ױ�������2�֣�
��CH2=CH2
 BrCH2CH2Br
BrCH2CH2Br
 ��2�֣�д��һ�������1�֣�����±�ص���Ҳ���ԣ�
��2�֣�д��һ�������1�֣�����±�ص���Ҳ���ԣ��������������Ϣ֪AΪ
 ��BΪ
��BΪ ����X�ṹ����֪CΪHOOCCOOH��
����X�ṹ����֪CΪHOOCCOOH����1��A�к����ǻ����������ֹ����ţ��١��ݷֱ�Ϊ�ӳɷ�Ӧ��������Ӧ��������Ӧ��������Ӧ��ȡ����Ӧ��
��2����Ӧ���Ҷ��������������Ҷ�ȩ��ˮ��
��Ӧ��
 ��HOOCCOOH����
��HOOCCOOH���� ��ˮ��
��ˮ����3�������������ֲ�ͬ������H˵������ȡ����λ�ڶ�λ��һ��ȡ������N����C��һ����C����N����ˮ��˵��������������N�Ļ���ֻ����-NH2����������Ľṹ��ʽ��
 ��
�� ��
�� ��
����4����DΪ
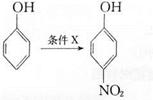
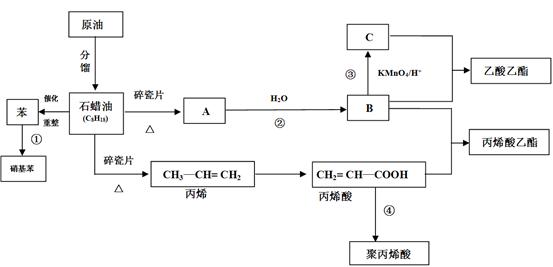
 ����Ũ���ᡢŨ������������·���������Ӧ����
����Ũ���ᡢŨ������������·���������Ӧ���� ������I����CH3I�������ǻ��������������Է���II�����С���ģ����ϢI��֪����±�����ܷ���ȡ����Ӧ���������ϳ�
������I����CH3I�������ǻ��������������Է���II�����С���ģ����ϢI��֪����±�����ܷ���ȡ����Ӧ���������ϳ� �����ñ�����XCH2CH2X��Ӧ����XCH2CH2X������ϩ��X2�ӳ��Ƶá�
�����ñ�����XCH2CH2X��Ӧ����XCH2CH2X������ϩ��X2�ӳ��Ƶá�
��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�
�����Ŀ


 ͨ������·�߿ɺϳɣ�II����
ͨ������·�߿ɺϳɣ�II����
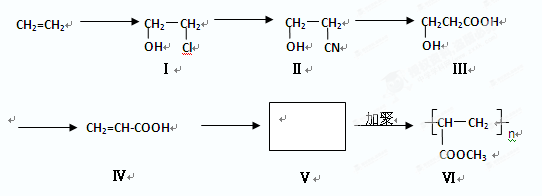
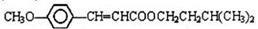 .�ϳ�·�����£�
.�ϳ�·�����£�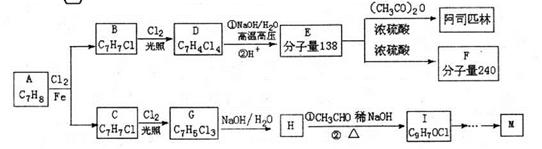
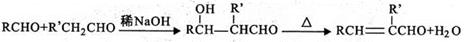
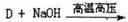 ____��E��F____
____��E��F____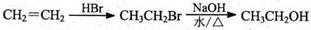




 B ________________________________________________
B ________________________________________________