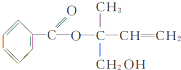��Ŀ����
����Ŀ��ij����ѧϰС�����۱�������˵����������ȷ˵������ĿΪ
�����������ﶼ�ǽ����������������������
��AlCl3��Һ��Al(OH)3����ı������������Ƿ��ж����ЧӦ
�����涨0.024kg12C���е�ԭ����Ϊ�����ӵ����������״����44gCO2�����ԼΪ44.8L
����״���£���������Ĵ�Сȡ����������Ŀ�����Ӽ��ƽ������
��ͬ��ͬѹ�£��������Ķ�����������Ͷ�����̼�����ܶȱ�Ϊ16��11�������Ϊ11��16
����0.05mol��������������뵽100ml 1.0vmol/L�����У���Һ�����Ի�������
�����ý����ء��ơ��ơ�þ�������ɵ���Ӧ�������������Ƶ�
���Ͻ��۵㡢Ӳ�ȶ����ڳɷֽ�������ͭ��ʹ������ĺϽ𣬸������������;���ĺϽ�
��ͬ�������壺C60��12C�����ʯ��ʯī
����ҵ�ϳɰ����˹��̵�����Ҫ��������ҵ����������ˮ�ࡢұ��������Ҫ�õ���ԭ����ʯ��ʯ
A��3 B��5 C��4 D .7
���𰸡�A
��������
��������������������ﶼ�ǽ��������������һ�����������������Ϊ(CH3CO)2O��������AlCl3��Һ��Al(OH)3����ı����������ڷ�ɢ�����ӵĴ�С�����������涨0.024kg12C���е�ԭ����Ϊ�����ӵ����������״����44gCO2�����ʵ���Ϊ![]() =0.5mol������Ħ�����Ϊ44.8L/mol�������涨0.024kg12C���е�ԭ����Ϊ�����ӵ����������״����44gCO2�����ԼΪ22.4L��������Ӱ����������������ӵĴ�С�����Ӽ�ľ����Լ���Ŀ���٣���״��������ķ��Ӽ������ȣ������Ӽ����Զ���ڷ��ӵĴ�С����������������С������Ϊ������Ŀ�Ķ��٣�������ͬ��ͬѹ�£�������ܶ�֮�ȵ�����Է�������֮�ȣ�������������Ͷ�����̼�����ܶȱ�=
=0.5mol������Ħ�����Ϊ44.8L/mol�������涨0.024kg12C���е�ԭ����Ϊ�����ӵ����������״����44gCO2�����ԼΪ22.4L��������Ӱ����������������ӵĴ�С�����Ӽ�ľ����Լ���Ŀ���٣���״��������ķ��Ӽ������ȣ������Ӽ����Զ���ڷ��ӵĴ�С����������������С������Ϊ������Ŀ�Ķ��٣�������ͬ��ͬѹ�£�������ܶ�֮�ȵ�����Է�������֮�ȣ�������������Ͷ�����̼�����ܶȱ�=![]() =
=![]() ���������Ķ�����������Ͷ�����̼����������=
���������Ķ�����������Ͷ�����̼����������=![]() ��
��![]() =
=![]() ����ȷ������0.05mol��������������뵽100ml 1.0vmol/L����������Ӧ����ǿ������Ȼ�������Һ�����Ի�����������ȷ�������ý����ء��ơ��ơ�þ������Ӧ�������������Ƶ����������Ͻ��۵����ڳɷֽ�������Ӳ�������ɷֽ�������ͭ��ʹ������ĺϽ𣬸������������;���ĺϽ��������� 12C����̼Ԫ�صĵ��ʣ���������ҵ�ϳɰ����˹��̵�����Ҫ����������ͨ������ԭ�ϣ�ʯӢɰ��ʯ��ʯ������ȣ��ƹ�����ˮ���ԭ�ϣ�ʯ��ʯ�������ұ��������ԭ�ϣ�����ʯ����̿��ʯ��ʯ������Ҫ��ʯ��ʯΪԭ�ϣ���ȷ����ȷ����3������ѡA��
����ȷ������0.05mol��������������뵽100ml 1.0vmol/L����������Ӧ����ǿ������Ȼ�������Һ�����Ի�����������ȷ�������ý����ء��ơ��ơ�þ������Ӧ�������������Ƶ����������Ͻ��۵����ڳɷֽ�������Ӳ�������ɷֽ�������ͭ��ʹ������ĺϽ𣬸������������;���ĺϽ��������� 12C����̼Ԫ�صĵ��ʣ���������ҵ�ϳɰ����˹��̵�����Ҫ����������ͨ������ԭ�ϣ�ʯӢɰ��ʯ��ʯ������ȣ��ƹ�����ˮ���ԭ�ϣ�ʯ��ʯ�������ұ��������ԭ�ϣ�����ʯ����̿��ʯ��ʯ������Ҫ��ʯ��ʯΪԭ�ϣ���ȷ����ȷ����3������ѡA��