��Ŀ����
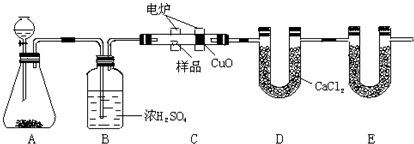
��ѧ�ϳ���ȼ�շ�ȷ���л������ɡ���ͼװ������ȼ�շ�ȷ���л������ʽ���õ�װ�ã����ַ������ڵ�¯����ʱ�ô�������������Ʒ�����ݸ����������ȷ���л������ɡ���E������Ӧװ�ã� 
����������⣺
��1��Aװ���з�Һ©��ʢ���������____________��д���йط�Ӧ�Ļ�ѧ����ʽ��_______
______________________��
��2��Cװ�ã�ȼ�չܣ���CuO��������________________��
��3��д��Eװ������ʢ�����ʵ�����________������������________________________��
��4������Bװ��ȥ�����ʵ�������ʲôӰ�죿_______________________________��
��5����ȷ��ȡ1.20 g��Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�E����������1.76 g��D����������0.72 g������л����ʵ��ʽΪ____________________��
��6��Ҫȷ�����л���ķ���ʽ������Ҫ____________________________________��
��1��H2O2����˫��ˮ�� 2H2O2![]() 2H2O+O2��
2H2O+O2��
��2��ʹ�û�������������CO2��H2O
��3����ʯ�һ��������� ����CO2����
��4����ɲ���л����к�����ƫ��
��5��CH2O
��6��֪���л������Է�������

��ϰ��ϵ�д�
 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ