��Ŀ����
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2(g)��O2(g)![]() 2H2O(l)����H����572 kJ��mol��1����ش��������⣺
2H2O(l)����H����572 kJ��mol��1����ش��������⣺
(1)�����������ܺ�________(����ڡ�����С�ڡ����ڡ�)��Ӧ�������ܺͣ�
(2)��2 mol������ȫȼ������ˮ��������ų�������________(����ڡ�����С�ڡ����ڡ�)572 kJ��
(3)��Ӧ2H2��O2��2H2O�������仯��ͼ��ʾ����֪��1 mol��H2��1 mol��O2��1 mol��H��O�еĻ�ѧ���ֱ���Ҫ����436 KJ��496 KJ��463 KJ��������Ӧ����(��)________(����ա��ų���)________KJ��

�𰸣�

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
 ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ�� ����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol
����ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-572kJ/mol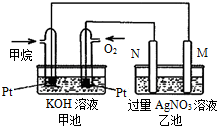 ��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��Ȼ������Ҫ�ɷּ���ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ���£���ش��������⣺CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��