��Ŀ����
��A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����ͼ����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·�ߡ�

|
|
|
|
|
|
��R��R����ʾ��������ԭ�ӣ�
��1��A�й����ŵĽṹ��ʽ�� ��
��2��B��C�Ļ�ѧ����ʽΪ ��
��3��E�ķ���ʽΪC4H8O�����й���E��˵����ȷ���� ������ĸ��ţ���
a. ��������Ʒ�Ӧ b. ������4��̼ԭ��һ����ƽ��
c. һ�������£�����Ũ�����ᷴӦ d. ��CH2=CHCH2OCH2CH3��Ϊͬϵ��
��4��G��H�漰���ķ�Ӧ������ ��
��5��I�ķ���ʽΪC4H6O2����ṹ��ʽΪ ��
��6��J��K�Ļ�ѧ����ʽΪ
��
��7��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��������˳���칹�������ǡ�OH����˫��̼�ϵĽṹ����
_______________________________________________________________��
![]() ��1�� C C ��1�֣�д�ɡ�C = C�����÷֣�
��1�� C C ��1�֣�д�ɡ�C = C�����÷֣�
|
��3��ac ��2�֣���ѡ��1�֣���ѡ����ѡ�֣�
��4���ӳɷ�Ӧ����ȥ��Ӧ����1�֣�
 ��5��CH3CH=CHCOOH ��2�֣�
��5��CH3CH=CHCOOH ��2�֣�
��6��n CH3CH=CHCOOCH3 ![]()
��2�֣�
![]()
![]() ��7��CH3CH=CHCH2OH CH2=C��CH2OH CH2=CH��CH��CH3
��7��CH3CH=CHCH2OH CH2=C��CH2OH CH2=CH��CH��CH3
��3�֣���1����1�֣���д1����1�֣���д1����1�֣�����Ϊֹ��

 ��������������������ϵ�д�
��������������������ϵ�д�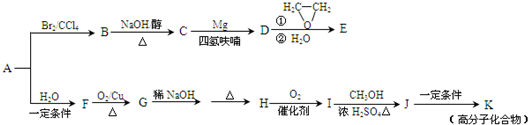









 +2��n-1��H2O
+2��n-1��H2O




