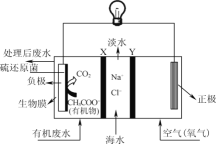
��Ŀ����
����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ�����(0.1000mol/L)���ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��ѡ�������ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ��������_______�����ʽ����ʽ�����ζ��ܵĻ���������ҡ����ƿ���۾�ע��______________________ֱ���������һ���������Һ�ɻ�ɫ��Ϊ_________ɫ����_________________________Ϊֹ��
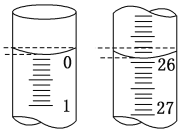
��2���ζ���ʼ�ͽ���ʱ�ζ��ܵ�Һ����ͼ��ʾ�����յ����Ϊ_____mL�������������Ϊ_____mL��
��3�������м��ּ�������������ۣ����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�����ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и�������ʹ����NaOH��Һ��Ũ����ֵ_________��
������ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�������ʹ����NaOH��Һ��Ũ����ֵ_______��
��4���й����ݼ�¼���£�
�ζ����� | ����Һ���(mL) | �����������mL�� | |
�ζ�ǰ������mL�� | �ζ��������mL�� | ||
��һ�� | 20.00 | 0.50 | 25.40 |
�ڶ��� | 20.00 | 0.00 | 25.10 |
�����������ݣ����������NaOH��Һ��Ũ��Ϊ__________________________________��
���𰸡���ʽ ��ƿ����Һ��ɫ�仯 �� ������ڲ���ɫ 25.90 25.90 ��Ӱ�� ƫ�� 0.1250mol/L
��������
��1����������к͵ζ��Ĺ淶�Բ������⣻
��2�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��3������c(��) =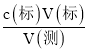 ��������������V(��)����Ӱ�죬�Դ��ж�Ũ�ȵ���
��������������V(��)����Ӱ�죬�Դ��ж�Ũ�ȵ���
��4������ͼ���������1��2��ƽ������V(����)���ٸ��������NaOH��Ӧ���c(NaOH)��
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��2����ʼ����Ϊ0.00mL���յ����Ϊ25.90mL��������Һ�����Ϊ(25.90-0.00)=25.90 mL��
��3�������ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и�������Һ�����ʵ������䣬��V(��)��Ӱ�죬��ʹ����NaOH��Һ��Ũ����ֵ���䣻
������ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V(��)ƫ�������c(��)= 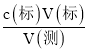 ��֪��ʹ�ⶨ���ƫ�ߣ�
��֪��ʹ�ⶨ���ƫ�ߣ�
��4�����ı�Һ���V(��)=![]() =0.0250L��c(��) =
=0.0250L��c(��) = 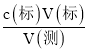 =
=![]() =0.1250mol/L��
=0.1250mol/L��

 �Ķ��쳵ϵ�д�
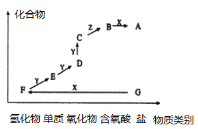
�Ķ��쳵ϵ�д�����Ŀ������A��D���鷴Ӧ�У���͢����ͬһ�����ӷ���ʽ��ʾ���ǣ� ��
ѡ�� | �� | �� |
A | �ѽ���������ϡ������ | �ѽ���������ϡ������ |
B | �Ȼ�����Һ�м���������NaOH��Һ | NaOH��Һ�м����������Ȼ�����Һ |
C | ϡ�����м�������������������Һ | ����������Һ�м���������ϡ���� |
D | ������Na2CO3��Һ��������HCl��Һ�� | ������HCl��Һ��������Na2CO3��Һ�� |
A.AB.BC.CD.D