��Ŀ����
��֪�����Ȼ�ѧ����ʽ��
��2H2��g��+O2 ��g��=2H2O��l������H=-570kJ?mol-1
��2H2��g��+O2 ��g��=2H2O��g������H=-483.6kJ?mol-1
��C��s��+
O2 ��g��=CO��g������H=-110.5kJ?mol-1
��C��s��+O2 ��g��=CO2��g������H=-393.5kJ?mol-1
�ش��������⣺
��1��H2��ȼ����Ϊ______��
��2��ȼ��1gH2����Һ̬ˮ���ų�������Ϊ______��
��3��д��COȼ�յ��Ȼ�ѧ����ʽ______��
��2H2��g��+O2 ��g��=2H2O��l������H=-570kJ?mol-1
��2H2��g��+O2 ��g��=2H2O��g������H=-483.6kJ?mol-1
��C��s��+
| 1 |
| 2 |
��C��s��+O2 ��g��=CO2��g������H=-393.5kJ?mol-1
�ش��������⣺
��1��H2��ȼ����Ϊ______��
��2��ȼ��1gH2����Һ̬ˮ���ų�������Ϊ______��
��3��д��COȼ�յ��Ȼ�ѧ����ʽ______��
��1�������Ȼ�ѧ����ʽ��2H2��g��+O2 ��g��=2H2O��l������H=-570kJ?mol-1��
���ȼ���ȵĸ����������õ�������ȼ���ȵ��Ȼ�ѧ����ʽΪ��H2��g��+
O2 ��g��=H2O��l������H=-285kJ?mol-1��
�ʴ�Ϊ��285KJ/mol��
��2��ȼ��1gH2����Һ̬ˮ���ų�������Ϊx�����������Ȼ�ѧ����ʽ���㣺
2H2��g��+O2 ��g��=2H2O��l������H=-570kJ?mol-1
4g 570KJ
1g x
x=142.5KJ��
�ʴ�Ϊ��142.5KJ��
��3�������Ȼ�ѧ����ʽ����C��s��+
O2 ��g��=CO��g������H=-110.5kJ?mol-1����C��s��+O2 ��g��=CO2��g������H=-393.5kJ?mol-1 ��
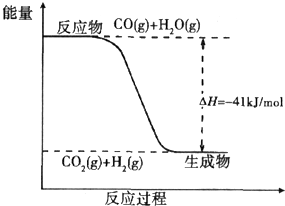
���ݸ�˹���ɢ�-�۵õ���CO��g��+
O2��g��=CO2��g����H=-283KJ/mol
�ʴ�Ϊ��CO��g��+
O2��g��=CO2��g����H=-283KJ/mol��
���ȼ���ȵĸ����������õ�������ȼ���ȵ��Ȼ�ѧ����ʽΪ��H2��g��+
| 1 |
| 2 |
�ʴ�Ϊ��285KJ/mol��
��2��ȼ��1gH2����Һ̬ˮ���ų�������Ϊx�����������Ȼ�ѧ����ʽ���㣺
2H2��g��+O2 ��g��=2H2O��l������H=-570kJ?mol-1
4g 570KJ
1g x
x=142.5KJ��
�ʴ�Ϊ��142.5KJ��
��3�������Ȼ�ѧ����ʽ����C��s��+
| 1 |
| 2 |
���ݸ�˹���ɢ�-�۵õ���CO��g��+
| 1 |
| 2 |
�ʴ�Ϊ��CO��g��+
| 1 |
| 2 |

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
 ú̿����ת��Ϊ�����Դ�ͻ���ԭ�ϣ�
ú̿����ת��Ϊ�����Դ�ͻ���ԭ�ϣ�