��Ŀ����
����Ŀ������ʮ���صij���F�Ľ��ͻ���ƽ���֮һΪ��ɫ����N2O4��N2O4��NO2ת�����Ȼ�ѧ����ʽΪ��N2O4(g)![]() 2NO2(g) ��H=+24.4KJ/mol
2NO2(g) ��H=+24.4KJ/mol
(1)��һ����N2O4Ͷ��̶��ݻ�����������У�����������˵����Ӧ�ﵽƽ����� ��
a��v��(N2O4)=2v��(NO2) b����ϵ��ɫ����
c������ƽ����Է����������� d�������ܶȲ���
�ﵽƽ�����������������¶ȣ��ٴε���ƽ��ʱ������������ɫ (����������dz�����䡱)���ж����� ��
��2)T��ʱ����1L�����ܱ�������Ͷ��1molCH4��1molH2O(g)��������Ӧ��CH4(g)+H2O(g)![]() CO(g)+3H2(g)������3min����Ӧ�ﵽƽ�⡣��֪ƽ��ʱc(CH4)=0.5mol/L
CO(g)+3H2(g)������3min����Ӧ�ﵽƽ�⡣��֪ƽ��ʱc(CH4)=0.5mol/L
��0��3min�ڣ��÷�Ӧ��ƽ����Ӧ����v(H2)=____________��
��T��ʱ���÷�Ӧ��ƽ�ⳣ��K=___________��
(3)��һ����������CO��H2�����Ƶü״���CH3OH��CO��ȼ����Ϊ��725.8 kJ/mol ��283.0 kJ/mol��1 molҺ̬ˮ�����̬ˮ����44.0 kJ��д���״�����ȫȼ������һ����̼����̬ˮ���Ȼ�ѧ����ʽ�� ��
(4)��ҵ����CO��H2�ڴ��������ºϳ�CH3OH,�䷴ӦΪ��CO(g)+2H2(g)![]() CH3OH(g)����n(CO) : n(H2)=1 : 2���ܱ������г��뷴Ӧ����ƽ��ʱ�������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ��
CH3OH(g)����n(CO) : n(H2)=1 : 2���ܱ������г��뷴Ӧ����ƽ��ʱ�������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ��
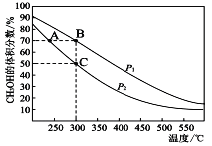
P1_________P2(�<����>����=��)
����C��ʱ��COת����Ϊ____________��
���𰸡�(15��)(1)bc(2��)������(1��)����Ӧ�����ȷ�Ӧ�������������䣬�¶�����ƽ�������ƶ���c(NO2)���ӣ���ɫ����(2��)
(2)��v(H2)=0.5moL/(L��min)(2��)����K=6.75(mol/L)2(2��)
(3)CH3OH(l)��O2(g)=CO(g)��2H2O(g)��H=-354.8kJ�Mmol(2��)
(4)��>(2��)����75%(2��)
��������
���������(1)a��Ӧ��2v��(N2O4)=v��(NO2) ʱ��Ӧ�ﵽƽ��״̬����a����b����ϵ��ɫ���䣬˵����������Ũ�Ȳ��䣬��Ӧ����ƽ��״̬����b��ȷ��c������������������䣬�淴Ӧ��С������������ʵ�������ƽ����Է���������С��������ƽ����Է�����������ʱ����Ӧ����ƽ��״̬����c��ȷ��d�������������������䣬�������ݻ����䣬�����ܶ�ʼ�ղ��䣬��d��������Ӧ�����ȷ�Ӧ�������������䣬�¶�����ƽ�������ƶ���c(NO2)���ӣ���ɫ���
(2)����1L�����ܱ�������Ͷ��1molCH4��1molH2O(g)��������Ӧ��CH4(g)+H2OCO(g)+3H2(g)����Ӧ��ʼc(CH4)=1molL-1������tmin����Ӧ�ﵽƽ�⣮��֪ƽ��ʱ��c(CH4)=0.5molL-1��v(CH4)=![]() =
=![]() mol/(Lmin)������֮�ȵ��ڻ�ѧ������֮�ȣ���v(H2)=3v(CH4)=3��
mol/(Lmin)������֮�ȵ��ڻ�ѧ������֮�ȣ���v(H2)=3v(CH4)=3��![]() mol/(Lmin)=
mol/(Lmin)=![]() mol/(Lmin)��
mol/(Lmin)��
��T��ʱ��1L�����ܱ�������
CH4(g)+H2O![]() CO(g)+3H2(g)
CO(g)+3H2(g)
��ʼ��(mol)�� 1 1 0 0
�仯��(mol)�� 0.5 0.5 0.5 1.5
ƽ����(mol)�� 0.5 0.5 0.5 1.5
ƽ�ⳣ��K=![]() =
= =6.75��
=6.75��
(3)��CO(g)��CH3OH(l)��ȼ������H�ֱ�Ϊ-283.0kJmol-1��-725.8kJmol-1����
��CO(g)+1/2O2(g)=CO2(g)��H=-283.0kJmol-1
��CH3OH(l)+3/2O2(g)=CO2(g)+2 H2O(l)��H=-725.8kJmol-1
��H2O(l)=H2O(g)��H=-44KJ/mol
�ɸ�˹���ɿ�֪����-��+����2�÷�ӦCH3OH(l)+O2(g)=CO(g)+2 H2O(g)���÷�Ӧ�ķ�Ӧ����H=-725.8kJmol-1-(-283.0kJmol-1)+2��44KJ/mol=-354.8kJmol-1��
(4)����300��ʱ������ѹǿ��ƽ�������ƶ���CH3OH�����������������p1��p2��
�������ܱ�����������1molCO��2molH2��CO��ת����Ϊx����
CO(g)+2H2(g)�TCH3OH(g)
��ʼ 1 2 0
�仯 x 2x x
���� 1-x 2-2x x
��C��ʱ��CH3OH���������=![]() =0.5�����x=0.75��
=0.5�����x=0.75��


