��Ŀ����
��ӦSO2(g)+ NO2(g)SO3(g)+NO(g) ,����һ���¶��£������ʵ���Ũ�Ⱦ�Ϊ2mol/L��SO2(g)��NO2(g)ע��һ�ܱ������У����ﵽƽ��״̬ʱ�����������SO2(g)��ת����Ϊ50%�������ڸ��¶��¡�
��1���˷�Ӧ��ƽ�ⳣ����
��2����SO2(g)�ij�ʼŨ������3mol/L, NO2(g)�ij�ʼŨ����Ϊ2mol/L����SO2(g)��NO2(g)��ת���ʱ�Ϊ���٣�
K=1��4�֣�ת����40%��60%��4�֣�
����:��

����ѧ������ѧ�뼼������15�֣����Ṥҵ����Ӧ�����ۺϾ���Ч�����⡣
��1�����������ĸ�������ѡ��һ���½�һ�����᳧������Ϊ��ַ��ѡ��
�Ľ�������ѡ��ı�ţ�
A���зḻ��������Դ�ij��� B��������������γ���
C����������϶�Ĺ�ҵ���� D���˿ڳ��ܵ��Ļ�����ҵ���ij���
��2���ݲ��㣬�Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1t 98%����������3.6��105kJ����������Ӧ:SO2(g)+1/2O2(g)=SO3(g) ��H=��98��3kJ��mol��1���ų��������������������еõ��������, ��ͨ�������ж�ÿ����1t98%����ֻ������ṩ��������������� ǧ��������
��3��CuFeS2�ǻ��������һ�ɷ֣�����ʱ��CuFeS2ת��ΪCuO��Fe2O3��SO2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4�������᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO3�ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
| ����¯�¶�/�� | 600 | 620 | 640 | 660 |
| ������CuSO4����������/% | 9.3 | 9.2 | 9.0 | 8.4 |
��֪CuSO4�ڵ���660��ʱ����ֽ⣬���Ҫ�����ϱ���CuSO4�������������¶����߶����͵�ԭ�� ��
 SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ������ʾ��
SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ������ʾ��
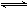 SO3(g)
K1,CO(g)+1/2O2(g)
SO3(g)
K1,CO(g)+1/2O2(g)  NO(g)��SO3(g)��һ�������½���ƽ�⣬�ټ���һ������O2������˵����ȷ����(����)
NO(g)��SO3(g)��һ�������½���ƽ�⣬�ټ���һ������O2������˵����ȷ����(����) NO(g) + SO3(g)��һ�������½���ƽ�⣬�ټ���һ������O2������˵����ȷ���� ��
��
NO(g) + SO3(g)��һ�������½���ƽ�⣬�ټ���һ������O2������˵����ȷ���� ��
��