��Ŀ����
�����ճ���������;��㡢�������Ľ������ϣ�
��1�������£�������������ʢװŨ�����ԭ����______��
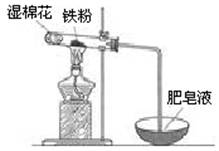
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��
��ʪ����������______���Թ��з�Ӧ�Ļ�ѧ����ʽ��______��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ������ȫ�ܽ⣬������Һ�д��ڵ���������______������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����壮���ⶨ�����к���SO2��CO2��H2��
������Ũ���ᷴӦ�Ļ�ѧ����ʽ��______��
�������л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ��______��
�۽�672mL����״�����ռ���������ͨ��������ˮ�У���Ӧ�����ӷ���ʽΪ______��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66g���ɴ���֪�ռ�����������SO2�����������______��
��1�������£�������������ʢװŨ�����ԭ����______��
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��
��ʪ����������______���Թ��з�Ӧ�Ļ�ѧ����ʽ��______��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ������ȫ�ܽ⣬������Һ�д��ڵ���������______������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����壮���ⶨ�����к���SO2��CO2��H2��
������Ũ���ᷴӦ�Ļ�ѧ����ʽ��______��
�������л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ��______��
�۽�672mL����״�����ռ���������ͨ��������ˮ�У���Ӧ�����ӷ���ʽΪ______��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66g���ɴ���֪�ռ�����������SO2�����������______��

��1�������£�����Ũ�����з����ۻ�����ʹ�������γ�һ�������ȶ�������Ĥ��������Ʒ��������ʢ��Ũ���ᣬ
�ʴ�Ϊ��Ũ����ʹ�������γ�һ�������ȶ�������Ĥ��
��2������Ϊ������ˮ�����ķ�Ӧ���Թ��������ۣ�����ʪ����Ҫ���ṩˮ���������ڸ�������ˮ��Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O
Fe3O4+4H2��
�ʴ�Ϊ���ṩˮ������3Fe+4H2O
Fe3O4+4H2��
������ˮ������Ӧ������Ϊ����������������������������ᣬ������ȫ�ܽ⣬�ط�����Ӧ��Fe3O4+8HCl=FeCl2+2FeCl3+4H2O������������Һ�д��ڵ���������һ����Fe2+��H+��������������ɫ�����п��ܺ��й������������ܽ�����������ȫ��ת���ɶ��������ӣ�Fe+2Fe3+�T3Fe2+������������Һ�д��ڵ���������һ����Fe2+��H+��������Fe3+��
�ʴ�Ϊ��b��
��3��������Ũ���ᷴӦ����������+3������Ũ���ᱻ��ԭ��+4�۵��������������Ͷ��������ˮ��
�ʴ�Ϊ��2Fe+6H2SO4��Ũ��
Fe2��SO4��3+3SO2��+6H2O��
���ڷ�Ӧ�Ĺ����У�̼���ʿ��Ժ�Ũ���ᷴӦ���ɶ�����̼��������������壬
�ʴ�Ϊ��C+2H2SO4��Ũ��
CO2+2SO2+2H2O��
��SO2���л�ԭ�ԣ�ͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr+H2SO4�����ӷ���ʽ����д���������е�����������������ӣ��������ӷ���ʽΪSO2+Br2+2H2O�T2Br-+SO42-+4H+�����ɵ����������Ȼ����������ɫ������������ϴ��Ȼ���ٹ�������ô��������ᱵ��������n��������壩=
=0.03mol��
SO2 ��BaSO4
1mol 233g
n 4.66g
n=0.02mol��
�� SO2�����������
=
��
�ʴ�Ϊ��
��
�ʴ�Ϊ��Ũ����ʹ�������γ�һ�������ȶ�������Ĥ��
��2������Ϊ������ˮ�����ķ�Ӧ���Թ��������ۣ�����ʪ����Ҫ���ṩˮ���������ڸ�������ˮ��Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O
| ||
�ʴ�Ϊ���ṩˮ������3Fe+4H2O
| ||
������ˮ������Ӧ������Ϊ����������������������������ᣬ������ȫ�ܽ⣬�ط�����Ӧ��Fe3O4+8HCl=FeCl2+2FeCl3+4H2O������������Һ�д��ڵ���������һ����Fe2+��H+��������������ɫ�����п��ܺ��й������������ܽ�����������ȫ��ת���ɶ��������ӣ�Fe+2Fe3+�T3Fe2+������������Һ�д��ڵ���������һ����Fe2+��H+��������Fe3+��
�ʴ�Ϊ��b��
��3��������Ũ���ᷴӦ����������+3������Ũ���ᱻ��ԭ��+4�۵��������������Ͷ��������ˮ��
�ʴ�Ϊ��2Fe+6H2SO4��Ũ��
| ||
���ڷ�Ӧ�Ĺ����У�̼���ʿ��Ժ�Ũ���ᷴӦ���ɶ�����̼��������������壬
�ʴ�Ϊ��C+2H2SO4��Ũ��
| ||
��SO2���л�ԭ�ԣ�ͨ��������ˮ�У�����SO2+Br2+2H2O=2HBr+H2SO4�����ӷ���ʽ����д���������е�����������������ӣ��������ӷ���ʽΪSO2+Br2+2H2O�T2Br-+SO42-+4H+�����ɵ����������Ȼ����������ɫ������������ϴ��Ȼ���ٹ�������ô��������ᱵ��������n��������壩=
| 0.672L |
| 22.4L/mol |
SO2 ��BaSO4
1mol 233g
n 4.66g
n=0.02mol��
�� SO2�����������
| 0.02 |
| 0.03 |
| 2 |
| 3 |
�ʴ�Ϊ��
| 2 |
| 3 |

��ϰ��ϵ�д�
 ��������������������ϵ�д�
��������������������ϵ�д�
�����Ŀ
 �����ճ���������;��㡢�������Ľ������ϣ�
�����ճ���������;��㡢�������Ľ������ϣ�