��Ŀ����
����Ŀ����1����ͬ���ʵ�����O2��O3��������Ϊ___________��
��2��2.5 mol Ba(OH)2���__________��OH-��2.5 mol Ba(OH)2��������___________g��
��3���ڱ�״���£�1.7 g������ռ�����ԼΪ___________L�������״��_________L���⺬����ͬ��Ŀ����ԭ�ӡ�
��4��ij��̬�����ﻯѧʽΪRO2���ڱ�״���£�1.28 g������������Ϊ448 mL������������Ħ������Ϊ_____________��
��5��ͬ��ͬѹ�£�SO2�뺤�����ܶ�֮��Ϊ___________����������ͬ����������������Ϊ___________
���𰸡� 2��3 5NA 427.5g 2.24 L 3.36 L 64 g/mol 16��1 1��16
��������(1)O2��Ħ������Ϊ32g/mol��O3��Ħ������Ϊ48g/mol����ͬ���ʵ�����O2��O3��������Ϊ32:48=2:3��
(2)�ɻ�ѧʽ��֪��2.5molBa(OH)2�к���5molOH-����N=nNA��֪OH-����Ϊ5NA=3.01��1024��Ba(OH)2��Ħ������Ϊ171g/mol��2.5 mol Ba(OH)2��������2.5 mol =427.5 g��
(3)1.7g �������ʵ���Ϊ![]() =0.1mol���������Ϊ0.1mol��22.4L/mol=2.24L���뺬����ͬHԭ����Ŀ����������ʵ���Ϊ
=0.1mol���������Ϊ0.1mol��22.4L/mol=2.24L���뺬����ͬHԭ����Ŀ����������ʵ���Ϊ![]() =0.15mol�����Ϊ0.15mol��22.4L/mol=3.36L��
=0.15mol�����Ϊ0.15mol��22.4L/mol=3.36L��
(4)����������ʵ���Ϊ![]() =0.02mol���������Ħ������Ϊ
=0.02mol���������Ħ������Ϊ![]() =64g/mol��
=64g/mol��
(5)��ͬ�����£��ܶ�֮�ȵ���Ħ������֮�ȣ���SO2�뺤�����ܶ�֮��=64g/mol�U4g/mol=16�U1����ͬ�����£�������ͬ���������֮����Ħ�������ɷ��ȣ�����������������=4g/mol�U64g/mol=1�U16��

����Ŀ����ͼ����ʾ��һЩ���ʻ�����Ĵ�����ϵ����ȷ����( )
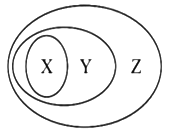
X | Y | Z | |
A | �ɱ� | ������ | ������ |
B | ��ˮ����� | ����� | ������ |
C | ������Һ | ���� | ��ɢϵ |
D | �û���Ӧ | ������ԭ��Ӧ | ���ӷ�Ӧ |
A. A B. B C. C D. D