��Ŀ����
���Ȼ���(TiCl4)����ȡ���캽�չ�ҵ���ϡ����ѺϽ����Ҫԭ�ϡ���������(��Ҫ�ɷ���FeTiO3)�Ʊ�TiCl4�Ȳ�Ʒ��һ�ֹ�������ʾ��ͼ���£�
(1)�����м�����м������Һ����ɫ����ʱ��Һ�Գ�ǿ���ԡ��ù����������·�Ӧ������
Fe��2Fe3��=3Fe2��
2TiO2��(��ɫ)��Fe��4H��=2Ti3��(��ɫ)��Fe2����2H2O
Ti3��(��ɫ)��Fe3����H2O=TiO2��(��ɫ)��Fe2����2H��
������������� ��
(2)�ڢڡ��۹��չ�������Ҫ�����������γ�TiO2��nH2O�ܽ������ܽ��ķ�ɢ�ʿ���ֱ����С�� ��Χ��
(3)���Ѣ����ƵõĹ���TiO2��nH2O������ϴ��ȥ���е����ʣ������Ƶ��Ѱۡ���֪25 ��ʱ��Ksp[Fe(OH)3]��2.79��10��39�����¶��·�ӦFe(OH)3��3H�� Fe3����3H2O��ƽ�ⳣ��K�� ��
Fe3����3H2O��ƽ�ⳣ��K�� ��
(4)��֪��TiO2(s)��2Cl2(g)=TiCl4(l)��O2(g) ��H����140 kJ��mol��1
2C(s)��O2(g)=2CO(g)����H����221 kJ��mol��1
д������TiO2�ͽ�̿��������Ӧ����Һ̬TiCl4��CO������Ȼ�ѧ����ʽ�� ��
(5)�������վ��гɱ��͡����õ�Ʒλ����Ϊԭ�ϵ��ŵ㡣������ɫ��ѧ����ù��������д��ڵIJ���֮���� (ֻҪ��д��һ��)��
(6)�����±���Ϣ��Ҫ���ƺ�����SiCl4���ʵ�TiCl4���ɲ��� ������
| | TiCl4 | SiCl4 |
| �۵�/�� | ��25.0 | ��68.6 |
| �е�/�� | 136.4 | 57.6 |
(1)ʹFe3����ԭΪFe2��������Ti3������Fe2����������
(2)10��9��10��7 m(������������)
(3)2.79��103
(4)TiO2(s)��2Cl2(g)��2C(s)=TiCl4(l)��2CO(g)����H����81 kJ��mol��1
(5)��������(������������)
(6)����(��������)
����

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д���I�����������ѳ�Ϊ��ǰ��δ����һ��ȫ���Կ��⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��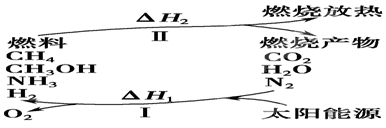
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O��O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
(II)��һ�Թ��м���0.01mol/L��KMnO4������Һ��0.1mol/LH2C2O4��Һ���ں����·������·�Ӧ��
2KMnO4+5 H2C2O4+3H2SO4��K2SO4+2MnSO4+10CO2+8H2O��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L��
��4���Լ���0��5�����ڣ���(H2C2O4)��____________��
(5)�����Ӧ�ӿ�ʼ����һ��ʱ������ʡ�ʱ��ͼ����ͼ��
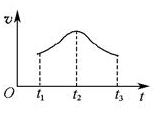 ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________�� ��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
��Ӧ��CO(g)+1/2O2(g) CO2(g)����H=bkJ��mol-1;
CO2(g)����H=bkJ��mol-1;
��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
(1)��500 ��ʱ���з�Ӧ��,CO2����ʼŨ��Ϊ2 mol��L-1,CO��ƽ��Ũ��Ϊ����������
(2)��Ӧ��Ϊ��������(ѡ����ȡ����ȡ�)��Ӧ��
(3)700 ��ʱ��Ӧ�ٴﵽƽ��״̬,Ҫʹ��ƽ�������ƶ�,������������ʱ,���Բ�ȡ�Ĵ�ʩ����������(�����)��
A.��С��Ӧ����� B.ͨ��CO2
C.�¶����ߵ�900 �� D.ʹ�ú��ʵĴ���
E.����Fe����
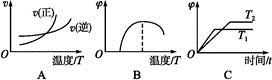
(4)����ͼ����Ϸ�Ӧ�ٵ�����������(�����)(ͼ��vΪ����,��Ϊ�������CO����,TΪ�¶���T1>T2)��
(5)�ɷ�Ӧ�ٺ͢ڿ����,��Ӧ2Fe(s)+O2(g)
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� (6)�����ø�˹����д��Fe(����)��O2(����)�����õ�Fe2O3(����)���Ȼ�ѧ����ʽ:�� ��
�Ҵ����ͺ�������35%,ʹȼ��ȼ�ո��ӳ��,ʹ�ó����Ҵ����ͣ�β���ŷŵ�CO��̼�⻯����ƽ������30%����,��Ч�Ľ��ͺͼ������к���β���ŷš���������ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���NOx����Ч������Ϊ�����������Ҫ���⡣NOx��������У��γ����꣬��ɿ�����Ⱦ��NOx����һ�ֺ���ɫ���壬������ˮ�ķ���ʽ�� ��
��2����֪NO2��N2O4�Ľṹʽ�ֱ��� ��
�� ��
��
| ���� | NO2 | N2O4 | |
| ��ѧ�� | N��O | N��N | N��O |
| ���ܣ�kJ/mol�� | 466 | 167 | 438 |
д��NO2ת��N2O4���Ȼ�ѧ����ʽ ��
��3���о���Ա������β��ϵͳ��װ�ô�ת����������Ч����NOx���ŷš�
�� д����CO��ԭNO����N2�Ļ�ѧ����ʽ ��
�� ��ʵ������ģ�´˷�Ӧ����һ�������µ��ܱ������У����NOת��ΪN2��ת�������¶ȱ仯�����n (NO)/n(CO)�����仯�������ͼ��

Ϊ�ﵽNOת��ΪN2�����ת���ʣ�Ӧ��ѡ�õ��¶Ⱥ�n(NO)/n(CO)�����ֱ�Ϊ �� ���÷�Ӧ��?H 0�����������������������
��4���� CxHy(��)����ԭNOxҲ����������������������Ⱦ�����ʡ�CH4��NO������Ӧ�Ļ�ѧ����ʽΪ ��
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
(1)ʵ���ã�1 g�״��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�22.7 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ__________________________________
(2)��֪��ӦCH3��CH3(g)�D��CH2=CH2(g)��H2(g)���йػ�ѧ���ļ������¡�
| ��ѧ�� | C��H | C=C | C��C | H��H |
| ����/kJ��mol��1 | 414.4 | 615.3 | 347.4 | 435.3 |
�Լ���÷�Ӧ�ķ�Ӧ��___________________________
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�����������㡣�����������Ȼ�ѧ����ʽ�����㷴Ӧ2C(s)��2H2(g)��O2(g)=CH3COOH(l)���ʱ䦤H��________��
��CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��1/2O2(g)=H2O(l)
��H3����285.8 kJ��mol��1
̼�͵��Ļ���������������������������ء�
��1����һ���¡������ܱ������з�����Ӧ�� Ni(s)+4CO(g) Ni(CO)4(g)��
Ni(CO)4(g)�� H<0�����ø÷�Ӧ���Խ�����ת��Ϊ���ȴ�99��9���ĸߴ������Ը÷�Ӧ��˵����ȷ���� (����ĸ���)��
H<0�����ø÷�Ӧ���Խ�����ת��Ϊ���ȴ�99��9���ĸߴ������Ը÷�Ӧ��˵����ȷ���� (����ĸ���)��
| A������Ni���������CO��ת���ʣ�Ni��ת���ʽ��� |
B����С�����ݻ���ƽ�����ƣ� H��С H��С |
| C����Ӧ�ﵽƽ�����CO�ٴδﵽƽ��ʱ��CO������������� |
| D����4v[Ni(CO)4]=v(CO)ʱ�������л�������ܶȲ���ʱ������˵����Ӧ�Ѵﻯѧƽ��״̬ |
��֪��C(s)+
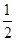 O2(g)=CO(g)
O2(g)=CO(g)  H=-Q1 kJ
H=-Q1 kJ mol-1
mol-1C(s)+ O2(g)=CO2(g)
 H=-Q2 kJ
H=-Q2 kJ mol-1
mol-1S(s)+O2(g)=SO2(g)
 H=-Q3 kJ
H=-Q3 kJ mol-1
mol-1��SO2(g)+2CO(g)=S(s)+2CO2(g)
 H= ��
H= ����3������������ɱ�һ����̼��ԭ���ɽ������ʺͶ�����̼��ͼ28��3�������ֽ��������Cr2O3��SnO2��PbO2��Cu2O)��һ����̼��ԭʱ
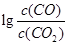 ���¶ȣ�t���Ĺ�ϵ����ͼ��
���¶ȣ�t���Ĺ�ϵ����ͼ��700oCʱ���������ѱ���ԭ�Ľ����������� (�ѧʽ)����һ����̼��ԭ�ý���������ʱ������Ӧ����ʽϵ��Ϊ��������ȣ��÷�Ӧ��ƽ�ⳣ��(K)��ֵ���� ��
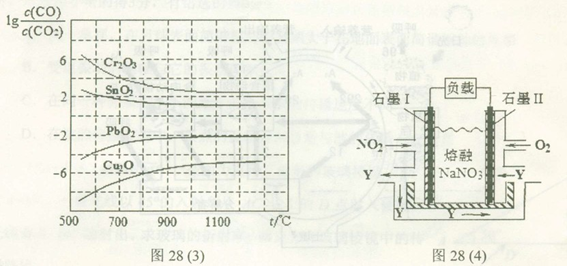
��4��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ������ͼ28��4����ʾ���õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦʽΪ ��
����ȼ�ϵ��ʹ��һ��ʱ����ռ���20mol Y������������Ҫ���ı�״�������������Ϊ L��
��Ԫ�صĻ���������࣬����Ҳ������ͬ��
��1��NO2�н�ǿ�������ԣ��ܽ�SO2��������SO3����������ԭΪNO����֪��������Ӧ�����������仯��ͼ��ʾ��
��NO2����SO2���Ȼ�ѧ����ʽΪ_________________________________��
��2����2L�ܱ������з���1mol��������һ���¶Ƚ������·�Ӧ��
2NH3(g) N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
| ʱ��t/min | 0 | 1 | 2 | 3 | 4 | 5 |
| ��ѹǿp 100 kPa | 5 | 5.6 | 6.4 | 6.8 | 7 | 7 |
��ƽ��ʱ������ת����Ϊ___________��
��3���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ��ڿ�������ȫȼ�����ɵ���������Ӧת��0.2mol����ʱ�����������ڱ�״���µ����Ϊ______________����������ˮ���Է����백ˮ���Ƶĵ��룬��д��������ˮ��Һ�еĵ��뷽��ʽ��
__________________��дһ�����ɣ���
��4��NH4+����Һ���ܷ���ˮ�ⷴӦ����25��ʱ��0.1mol/L�Ȼ����Һ��ˮ�������������Ũ��Ϊ1��10-5 mol/L�����ڸ��¶��´���Һ�а�ˮ�ĵ���ƽ�ⳣ��Kb��NH3��H2O��=__________________��

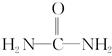 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ�� 3AlCl(g)+3CO(g)����H="a" kJ��mol-1
3AlCl(g)+3CO(g)����H="a" kJ��mol-1