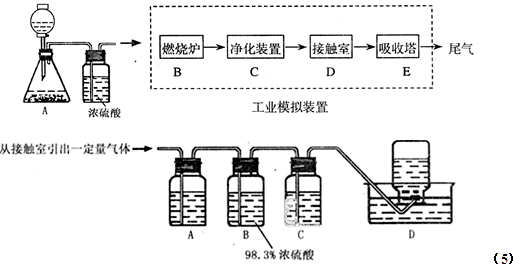
��Ŀ����
ijͬѧ����������Ϊ98�����ܶ�Ϊ1.84 g��cm3��Ũ���ᣬ����100 mL 2 mol��L H2SO4��Һ���������йص�ʵ�顣�Իش��������⣺
��1����������Ũ����������
��2��������������ѡ��ʵ������Ҫ������ (�����)��
| A��10 mL��Ͳ | B��20 mL��Ͳ | C��100 mL�ձ� | D��100 mL����ƿ |
��3����ͬѧΪ�ⶨij̼������Ʒ�Ĵ��ȣ�ȡ2.5 g��̼������Ʒ��������������ϡ���ᡣ̼������ȫ��Ӧ�����ʲ���Ӧ�������ɶ�����̼����448mL����״���������̼������Ʒ��Na2CO3������������
��1��10.9mL��3�֣�
��2��B��C��D��G��I����1�֣���5�֣�
��3��84.8����2�֣�
����

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
�����Ŀ