��Ŀ����
13����25��ʱ�������ܱ�������X��Y��Z��������ij�ʼ���ʵ�����ƽ�����ʵ������£�| ���� | X | Y | Z |
| ��ʼ���ʵ���/mol | 0��1 | 0��3 | 0 |
| ƽ�����ʵ���/mol | 0��05 | 0��2 | 0��1 |
| A�� | ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50% | |
| B�� | ��Ӧ�ɱ�ʾΪX+2Y?2Z | |
| C�� | ����ʼʱX��Y��Z�����ʵ����ֱ�Ϊ0.1 mol��0.4mol��0.2mol����ƽ��ʱ��Z���������һ������ | |
| D�� | ����ʼʱX��Y��Z�����ʵ����ֱ�Ϊ0.05 mol��0.15mol��0.1mol����ƽ��ʱ��X��ת����һ����ԭͶ����� |
���� A������ת����=$\frac{���ʵ����仯��}{��ʼ���ʵ���}$��100%���㣻
B���������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ݴ��жϻ�ѧ����ʽ��
C����ЧΪ��ԭƽ��Ļ�����ѹǿ����һ�������ƽ���ƶ��жϣ�
D����ЧΪ��ԭƽ��Ļ����Ͻ�Y��С0.5mol��ƽ�����淴Ӧ�����ƶ����ݴ˷�����
��� �⣺A����Ӧ�ﵽƽ��ʱ��X��ת����Ϊ��$\frac{0.1mol-0.05mol}{0.1mol}$��100%=50%����A��ȷ��
B����ѧ������֮�ȵ������ʵ����仯��֮�ȣ����ݱ��е����ݿ�֪��X��Y�����ʵ�����С������Ϊ��Ӧ�Z�����ʵ������ӣ�Ϊ�������n��X��������n��Y������n��Z����=0.05��0.1��0.1=1��2��2����Ӧ�ķ���ʽΪX+2Y?2Z����B��ȷ��
C�����ݷ�ӦX+2Y?2Z����0.2molZȫ��ת��ΪX��Y����X��Y����ʼ���ʵ���Ϊ0.2mol��0.6mol����ЧΪ��ԭƽ��Ļ�����Ũ������һ����ƽ��������Ӧ�����ƶ���ƽ��ʱZ�������������C��ȷ��
D�����ݷ�ӦX+2Y?2Z����0.1molZȫ��ת��ΪX��Y����X��Y����ʼ���ʵ���Ϊ0.1mol��0.25mol����ЧΪ��ԭƽ��Ļ����Ͻ�Y��С0.5mol��ƽ�����淴Ӧ�����ƶ�����X��ת�����½�����D����
��ѡD��
���� ���⿼�黯ѧƽ����㡢��ѧƽ��Ӱ�����ء���Чƽ��ȣ����ؿ���ѧ����֪ʶ�����⣬�Ѷ��еȣ�

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�| A�� | ͬ������H2��Cl2��H2�ķ�����һ����Cl2�� | |
| B�� | 0.5mol���������0.5g | |
| C�� | Ħ�����������������Ӷ��ٵ�һ�������� | |
| D�� | 0.1mol H2SO4������ԭ�����ľ�ȷֵΪ1.204��1023 |
 CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�������1����CO2��NH3Ϊԭ�Ϻϳɻ������ص���Ҫ��Ӧ���£�
��2NH3��g��+CO2��g��=NH2CO2NH4��s������H=-159.47kJ•mol-1
��NH2CO2NH4��s��=CO��NH2��2��s��+H2O��g������H=a kJ•mol-1
��2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��g������H=-86.98kJ•mol-1
��aΪ+72.49kJ��mol-1��
��2����ѧ��������ù�ҵ�����е�CO2��ȡ�״���CO2+3H2CH3OH+H2O��
�Ƶõ�CH3OH������ȼ�ϵ�ص�ȼ�ϣ�
����KOH�����У������ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
�������ʵ�KOH�����õ��K2SO4��Һ�ķ����Ƶã���KOH��D���ڵõ��������ĵ缫��Ӧʽ�ǣ�4OH-+4e-=2H2O+O2��
��3������CO��H2��Ӧ�ɺϳ�CH3OCH3��
��֪��3H2��g��+3CO��g��=CH3OCH3��g��+CO2��g������H=-247kJ/mol
��һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��AE��
A�����¸�ѹ B��������� C�����������뺤��D������CO��Ũ�� E�������������
��4��CH3OCH3Ҳ����CH3OH�ϳɣ�
��֪��Ӧ2CH3OH��g��=CH3OCH3��g��+H2O��g������ij�¶��£���1L�ܱ������м���CH3OH����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�������
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.01 | 0.2 | 0.2 |
�ڸ��¶��µ�ƽ�ⳣ��Ϊ400��
����ƽ��������������ټ���0.01mol CH3OH��0.2mol CH3OCH3����ʱ�����淴Ӧ���ʵĴ�С��v���� v�� �����������������=������
| A�� | �۳� | B�� | �����ЧӦ | C�� | �����˶� | D�� | ������ |
| A�� | SO42-? | B�� | Na+ | C�� | Cl- | D�� | CH3COO- |
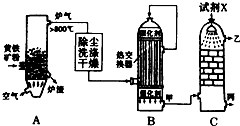 �ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺
�ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺