��Ŀ����
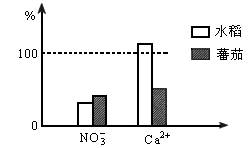
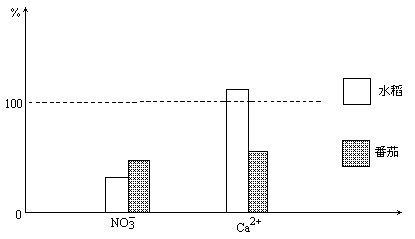
��ˮ���ͷ��ѷֱ������ں�����ͬŨ�ȵ�Ca(NO3)2������Ӫ��Һ�У��������е��뼸�η�̪��Һ�������鿪ʼʱ��Һ�е�NO3-��Ca2+��Ũ��ֵΪ100%��һ��ʱ����֪����Ӫ��Һ��Ca2+��NO3-Ũ�ȵİٷֱȱ仯��ͼ��ʾ��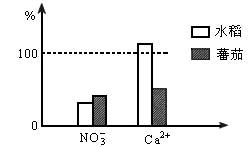
��������Ͻ��������
��1��NO3-��Ca2+Ũ���½���ԭ����________��ˮ����Ӫ��Һ��Ca2+Ũ�����ߵ�ԭ����________��
��2��ֲ���NO3-�����ն���Ca2+����ԭ����________��
��3���������ʵ���������������������Һ����ɫ�仯����д������ñ仯�Ļ�ѧ����ʽ��
������
| ���� �������ؿ��飺��ֲ���ˮ�����պͿ����������յ�֪ʶ�����ߵĹ�ϵ���ۺ��������ڶ�ʵ��ķ�������ͼ���������������ۻ�ѧ���й�ˮ��Ļ���֪ʶ��
ֲ��������������ͨ����ϵ���ϴ�������Һ������ˮ�ֺͿ������ӣ�������ˮ�ֺͿ�����������������Զ����Ĺ��̣������ǵ�����ǿ�ȿ��ܲ�ͬ���Կ������ӵ����վ���ѡ���ԣ������յĶ���ȡ���ڸ�ϸ��Ĥ�������������������Կ������ӵ�������ͨ�����ӽ���������ʵ�ֵģ�����ϸ��ͨ���������ò���CO2��CO2����ˮ����H2CO3��H2CO3�T�TH++HCO3-��H+��HCO3-���������е�Ca2+��NO3-����������ʹ�������ӽ����ϸ����H+��HCO3-����������Һ�У����ܻ�����������ҺpH�ı仯�� �� ��1��ֲ�����������в������տ������� ˮ����ˮ�ֵ�����ǿ�ȴ����ڶ�Ca2+������ǿ�� ��2��ֲ���ϸ��Ĥ������NO3-��������Ŀ��������Ca2+��������Ŀ ��3������ɺ�ɫ��HCO3-+H2O�T�TH2CO3+OH-��
|