��Ŀ����
������Ȼ�������¹����緢������Ȼ�������������壬������ѧ����֪ʶ�ش��������⣺
��1��Ϊ��ֹ�����¹��и������Ա������������Ա�����õ��ȼ�շ�������ȥ�ж����壬��д����Ӧ�Ļ�ѧ����ʽ
��2����ʯ���꾮�У�Ϊ��ֹ�����¹ʣ�Ҫ���ݾ���ѹǿ�����ؾ�ʯ���ܶ�4.5g/cm3���ң����ཬ��һ��������Ϻ���뾮�С�д���ؾ�ʯ�Ļ�ѧʽ ��˵���ؾ�ʯ����ѹ�����ϵ�ԭ��
��3����������꾮Һ��pHֵӦ����9.5���ϣ���������������Һ������Һ��д��������Ӧ�Ļ�ѧ����ʽ
��1��Ϊ��ֹ�����¹��и������Ա������������Ա�����õ��ȼ�շ�������ȥ�ж����壬��д����Ӧ�Ļ�ѧ����ʽ
��2����ʯ���꾮�У�Ϊ��ֹ�����¹ʣ�Ҫ���ݾ���ѹǿ�����ؾ�ʯ���ܶ�4.5g/cm3���ң����ཬ��һ��������Ϻ���뾮�С�д���ؾ�ʯ�Ļ�ѧʽ ��˵���ؾ�ʯ����ѹ�����ϵ�ԭ��
��3����������꾮Һ��pHֵӦ����9.5���ϣ���������������Һ������Һ��д��������Ӧ�Ļ�ѧ����ʽ
��1��2H2S + 3O2 �� 2SO2 + 2H2O
��2��BaSO4 �� �ܶȴ�
��3��H2S + 2NaOH �� Na2S + H2O
��2��BaSO4 �� �ܶȴ�
��3��H2S + 2NaOH �� Na2S + H2O
��

��ϰ��ϵ�д�
�����Ŀ
 2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��
2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��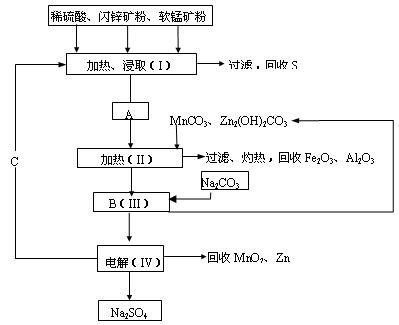
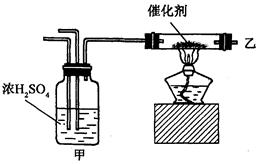
 ��
�� ��
��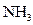
 2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺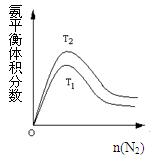
 2(g)+CO(g) ��ƽ�ⳣ��K1
2(g)+CO(g) ��ƽ�ⳣ��K1

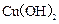 ��ʹ��Һ�ָ���ԭ��Ũ��
��ʹ��Һ�ָ���ԭ��Ũ��