��Ŀ����
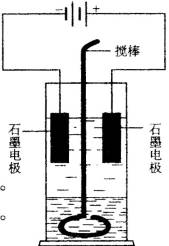 �ڲ���ԲͲ��ʢ��������ɫ�Ļ������ܵ�����Һ�塣�ϲ�Һ���в�������ʯī�缫��ԲͲ�ڻ�����һ���¶���ɻ�״�IJ����������������½���Һ�壬װ������ͼ����ͨ��Դ��������Χ��Һ�������ɫ������ɫ��dz������������������ɡ�ֹͣͨ�磬ȡ���缫���ý������¾��ҽ��������ú�Һ���ֳַ����㣬�²�Һ����Ϻ�ɫ���ϲ�Һ�弸����ɫ����������ʵ��ش�
�ڲ���ԲͲ��ʢ��������ɫ�Ļ������ܵ�����Һ�塣�ϲ�Һ���в�������ʯī�缫��ԲͲ�ڻ�����һ���¶���ɻ�״�IJ����������������½���Һ�壬װ������ͼ����ͨ��Դ��������Χ��Һ�������ɫ������ɫ��dz������������������ɡ�ֹͣͨ�磬ȡ���缫���ý������¾��ҽ��������ú�Һ���ֳַ����㣬�²�Һ����Ϻ�ɫ���ϲ�Һ�弸����ɫ����������ʵ��ش�
��1�������ϵĵ缫��ӦʽΪ_____________________��
��2�������ϵĵ缫��ӦʽΪ_____________________��
��3��ԭ�ϲ�Һ����_____________________________��
��4��ԭ�²�Һ����______________________________��
��5�����������Һ����ɫ�����仯��ԭ����________����������������
���� ���������������������������������������������������������������� ��
��6��Ҫ�����ϲ�Һ���к��еĽ������ӣ��䷽����_____________________________��
������_____________________________________________________________
____________________________________________________________________��
�𰸣�
������
������
| ��1��������KClO3������Mg���������ȼ Fe
��2��Fe2O3��6HCl��2FeCl3��3H2O MgO��2HCl��MgCl2��H2O ��3��Fe��2H+��Fe2+��H2�� Fe��2Fe3+��3Fe2+
|

��ϰ��ϵ�д�
�����Ŀ


